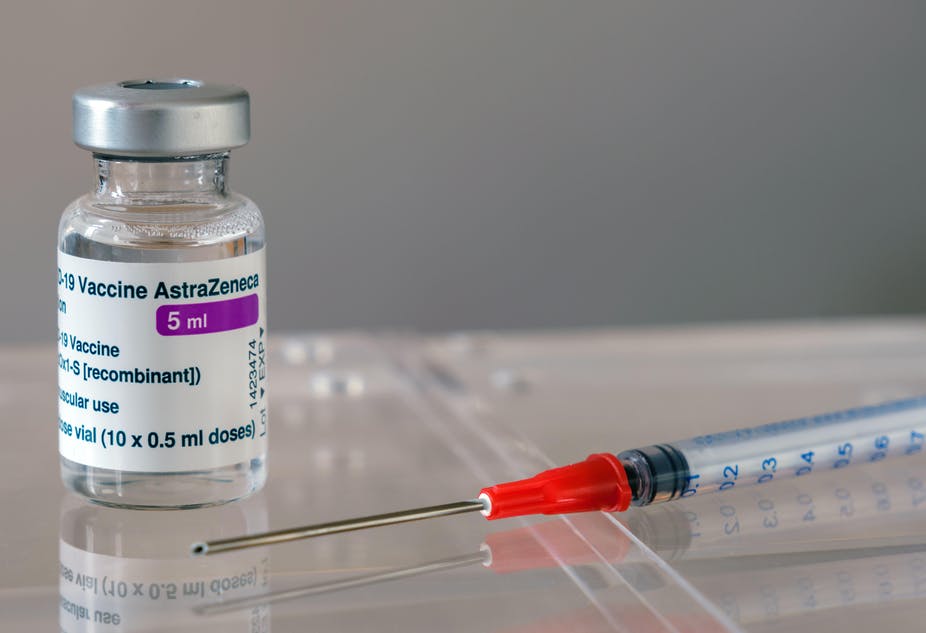
By the time cases of blood clotting in patients who had received the AstraZeneca vaccine had begun to emerge on the Continent (in March), Britain had already administered 11 million doses (the first ones having been given in January). No such adverse events had been reported publicly in Britain, but not for a lack of cases, according to the findings of a new investigation. Clotting cases were recorded in the UK’s Yellow Card database (a website for reporting adverse drug reactions) in January but were missed at first by the Medicines and Healthcare products Regulatory Agency (MHRA) – possibly due to the algorithms it uses to interrogate UK data. The Telegraph has the story.
On March 11th, the MHRA put out a statement saying it could see no evidence of a problem…
But the MHRA was, it appears, wrong. An investigation by the Telegraph has established that signals had been firing unnoticed in the UK’s Yellow Card database for at least a month, perhaps longer.
In January, a patient suffered a brain clot following their first dose of the AstraZeneca jab… Then in early February, two similar cases followed, including a death and a life-changing CVST clot in a young adult. All had low platelets and all were reported into the Yellow Card system.
On Friday, the MHRA told the Telegraph: “We are aware of thromboembolic events that occurred in January, however, our first report was received in the week commencing February 8th…. we cannot disclose information about individual cases to protect patient and reporter confidentiality.”
… The MHRA faces serious questions as to why it did not detect the signals sooner. The issue is not that it has been left looking flatfooted or even that earlier detection would necessarily have altered its advice, but that the delay left it unable to shape international policy and confidence in what remains a vital vaccine in the fight against Covid for the world.
Professor Stephan Lewandowsky, a psychologist at the University of Bristol studying the rollout of Covid vaccines, told the Financial Times on Friday: “The MHRA was slow in responding to the emergence of a specific constellation of symptoms associated with the AstraZeneca vaccine and slow to communicate what they were finding – and I am not the only one who thinks so.”
This slow repose was caused, it is said, by algorithms which were not as sensitive as the ones used by European health agencies to sift through data.
From January 4th to March 14th, a total of 532 “blood system events”, including 20 deaths, came through the UK’s Yellow Card system relating to the AstraZeneca jab, according to an analysis of published MHRA data by Dr Hamid Merchant, a pharmaceutical scientist at the University of Huddersfield. There were thousands of non-blood-related reports besides.
Of the thrombotic events recorded, four related to CVST (but no deaths were recorded), 55 were non-site specific and there were clusters of 64 and 66 cases in the lungs and deep veins respectively. There were then 267 general bleeding events and six deaths, three of which resulted from cerebral haemorrhage. Finally, there were 60 cases of thrombocytopenia, including two deaths.
To sift such data, regulators build algorithms that must balance “sensitivity” against leg-work. The more sensitive the algorithm, the more warning signals it will throw up to investigate – and many of those labour-intensive investigations will prove fruitless.
It is not known exactly what parameters the MHRA set but it is clear they were not as sensitive as those used by some regulators in Europe.
The MHRA says it followed a principle of applying “statistical techniques which can tell us if we are seeing disproportionately more cases than we would expect to see based on what is known about background rates of illness in the absence of vaccination”. This is reflected in the regulator’s initial statement when it said clotting reports were not above normal.
But other countries turned the sensitivity gauge up to 11. “Our policy is if it is associated with a death, or very serious adverse drug reaction, we will look into it right away,” David Benee Olsen, senior advisor at the Norwegian Medicines Agency, told the Telegraph.
Reports suggest that the MHRA was more rigorous in its examination of data relating to the Covid vaccine produced by Pfizer. The Telegraph clarifies that “there is no suggestion whatever that the MHRA covered up the reporting of CVST with thrombocytopenia – it just did not spot the still unproven issue as early as others”.
Worth reading in full.
Stop Press: Canada has reported a second case of rare blood clots with low platelets after vaccination with AstraZeneca’s Covid vaccine in a week.











To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
I think that Clean Renewables Action Plan might produce a more useful acronym.
Or ‘Surface Heat Inhibition Treatise’
Miliband’s
Energy
Reforms
Destroy
England
Problem solved. Well done!
Mad
Idiotic
Laughable
Lunatic
Illiterate
Bonkers
Anti-British
Nutty
Destroyer
Check the spelling of his surname, perhaps
Oh bother. I can’t help thinking “milli” when I think about his intelligence. I’ll have to cut out “laughable” or “lunatic”. Can’t decide which to ditch…
Mad
Idiotic
Lunatic
Illiterate
Bonkers
Anti-British
Nutty
Destroyer
It’s all a bit of a chimpanzee’s tea party, really, isn’t it….
‘Ocean-emitted dimethyl sulfide (DMS) is a major source of climate-cooling aerosols. However, most of the marine biogenic sulfur cycling is not routed to DMS but to methanethiol (MeSH), another volatile whose reactivity has hitherto hampered measurements. Therefore, the global emissions and climate impact of MeSH remain unexplored.
MeSH emissions increase the sulfate aerosol burden by 30 to 70% over the Southern Ocean
Accounting for MeSH emissions reduces the radiative bias of current climate models in this climatically relevant region.’
So the climate models also known as Surface Heat Inhibition Treatises (S.H.I.T.) are precisely that…..
And the self appointed ‘experts’ are only too well aware:
Andy Pitman, director of the Australian Research Council Centre of Excellence for Climate Extremes
‘We don’t actually know to what degree our terrestrial and marine systems will continue to support net-zero ambitions: they might turn out to be sources of CO2 and methane and undermine our net zero ambitions.’
He also admits that he and fellow climate alarmists have no idea
♦ when and where so-called “tipping points” might arise (wow, so honest!)
♦ whether climate change will increase or decrease the Murray Darling water flows
♦ whether an increase in CO2 will cause more or less rain for a given location
♦ how climate change will impact cities and urban landscapes (Andy, stop upsetting the Melbourne and Sydney city councils’ climate crusaders)
♦ how wind droughts and heavy clouding might undermine renewables and net-zero targeting (via week-long blackouts).
In a repudiation of the “settled science” notion the climate crowd has pushed for 25 years, Pitman now acknowledges that despite decades of study, the catastrophists still have no idea if Australia will see more El Nino, rather than La Nina, climate events, or even whether more vegetation will reduce or increase greenhouse emissions (so much for tree plantings offsetting emissions).
Other profound unknowns include, according to Pitman, include sea-ice extent, cloud processes, ice-sheet dynamics and urban and agricultural landscape impacts (p22).
He lets another cat out of the bag with news that between March 16 and 18 of this year, a giant 70-billion tonne snowfall hit East Antarctica, contributing to a net ice gain in Antarctica reversing the 20-year trend of ice loss.
Climate models remain too crude to handle the key processes of atmospheric rivers around the Antarctic which created that startling reversal
Pitman even concedes that current climate models can’t predict whether natural disasters will become more or less common in the warming era.
‘Climate predictions are built on very old science.’
https://www.science.org.au/supporting-science/science-advice-and-policy/decadal-plans-for-science/a-decadal-plan-for-australian-earth-system-science-2024-2033
Oh for heaven’s sake! Very expensive tea being drunk, all the while scrambling the knitting………
The Climate Nintendos are also wrong on rates of CO2 absorption by around 40% for plants and seas. And now it seems that broadleaved trees give off isoprene that at night seeds clouds. So much for the ‘settled’ science used in the gaming world.
It seems to me to be an absolute waste of time trying to engage with Miliband using science and reasoned argument to point out that the climate alarmism has no basis in fact. This isn’t because he is thick, cretinous or mad as some have observed – it is because he is carrying out his orders received from people with billions to spend in order to extract even more billions from British taxpayers; doubtless his reward will far exceed the traditional thirty pieces of silver. His blithe indifference to the suffering and anxiety his policies are causing points to his being a sociopath, certainly, and the sooner he is removed from post – along with anyone similarly afflicted – the better.
Correct. He expects to become very wealthy on the back of his destruction of the UK.
His brother is doing VERY nicely, by all accounts!
I am sure the Chinese are pumping a lot of investment into British energy infrastructure. All those green jobs are likely to be yellow jobs.
Our ‘thriving democracy’.
20% or less of the UK adult vote.
They don’t have a mandate for anything.
Yet here they are pursuing non-Green fascism, destroying the environment, our industry, our jobs and our national infrastructure.
By what authority?
Reminds me of the Convid Enabling act for the non-existent virus which destroyed my freedoms in 5 minutes.
Mass civil violence is going to be the only solution.
Mass civil disobedience. Not violence. Violence will play into their hands.
When people can’t stay warm, travel to work and feed themselves is when violence could start
Handy that there are lots of men of fighting age with no connection to Britain and its values who can be used to police any protests.
I don’t think they’ll follow orders if they are cold and hungry as well…
They will only do it if they get their Caliphate and rights to slaughter non-believers.
I’m afraid it is incumbent on the Great British Public to personally do everything they can to block the nonsense from proceeding. If that means contributing to the conditions which cause blackouts, so be it.
Don’t buy an EV. Crash the car market.
Don’t buy a heat pump. Use gas. If they won’t produce our own, make them import it.
Refuse a Smart meter; retain control of your own energy supply. You can keep your bills down if you want to without a gadget, using electricity, to show you.
Don’t change your diet.
Tell friends and acquaintances that the Net Zero project is unaffordable and based on climate lies. I talk to people in the supermarket queue when I get an opportunity – keep it lighthearted but get the message across.
Use cash. If they don’t know what you’re buying they can’t stop you from buying it.
Object to planning applications for windmills and solar farms. It probably won’t stop them, but we have to demonstrate that opposition is big, and growing.
Prepare: candles, matches, alternative sources of heating.
Join the Together Organisation.
This moron gave us the Climate Change Act in 2008 promising to save the planet and lower bills. Now here he is 16 years later still pretending to save the planet and still promising lower bills. But we currently have the highest electricity prices in the world and he is about to do more and more of the very same stuff. At the moment our electricity prices are 3 times higher than the USA, and Miliband will raise that to 5 or 6 times higher. When are the people actually going to wake up to the fact that you cannot run Industrial Society on wind and sun and if you attempt to do so you will do nothing but impoverish people and destroy the Industrial Base. —-All of the countries like Denmark, Germany and the UK who have the most wind turbines all have the highest prices, but Miliband is still pushing this idea that reality can be turned on its head. —-IT CAN’T.
Perhaps he’s trying to redefine “reality”?
Sadly Ratcliffe on Soar has closed but I presume yet to be blown up? Perhaps some AI lot could buy it to power a data centre and preserve it for the nation. Is that permitted I wonder? Anyway, as long as no more reliable generation is closed down we can probably get through to the end of the Student Union government in 2029 although we will be at risk of blackouts due to too much unreliables on the grid and some clouds arrive. Not much of Militwat’s plans will be delivered as there are not enough resources or the technology just doesn’t exist.
W_NKER !!!!…
Just asking – these fancy blades heading for the north sea – it’s pretty physically hostile out there – how long will they last and how much is built into replacement planning? Anybody out there willing to guess? Oh wait – Minibrain won’t be around to take the hit. Funny that.
“We’ve produced this at lightning pace – it’s packed with new steps towards clean power.”
Sound familiar? Remember Pfizer CEO Albert Bourla’s little gem? COVID-19 vaccine development is “moving at the speed of science”?Oh how they mock us.
Bastards all!
President Trump landslide election mandate makes Edstone Moribund irrelevant.
I suppose we all have to wait for the next, inevitable change in the weather back to cold, like it was in the ’60s and ’70s. Then the lunatics were predicting an ice-age.
The problem then will be that rectifying all the harm will cost a fortune, and de-industrialised Britain will be broke.
We’ve already been moving towards that for quite some time already. One of the reasons why climate politicals is getting ever more hysterical and destructive is that the people behind it know more about changing weather patterns than what their models generate: They know that they’re running out of time. So far, they’ve swapped the late 1980s/ 1990s invention called global warming for climate change to make the messaging a little more robust (German climate agitators have already claimed that very moderate amounts of snow in winter would be extreme weather caused by climate change) but they’re still relying on observable warming. But there ain’t any and simply inventing the numbers won’t work forever.
Maybe Milliband knows exactly what he is doing…
À superb article. It’s just a great pity that neither Milibrain, nor his henchpersons will ever read this for fear of becoming very, very frightened!
Yes, agreed, Ben Pile is superb, and fast becoming one of my favourite columnists.
‘The perfect storm is in fact the entirely predictable outcome of allowing ideological zombies bearing only false promises to set energy policy.’
I don’t know how they get away with it. A Milliband spokesman said today on Talk that we should not undermine and upset the excellent clever people working to solve the intermittency problem by complaining that it remains unsolved and that the current plan to rely on wind and sun for power is like a plan to commute in a car you know will not start on 2 random days a week, requiring you to have a taxi, meter running, on permanent standby.
Don’t harp on about it, it’s upsetting for the people working on the problem,
Can you believe it?
The taxi is gas-fired power, of course, on hot standby, which we will need for the foreseeable future If the lights are to stay on. So are we developing our gas reserves, which we possess in abundance? Oh, no. That would contravene our Paris commitments.
This isn’t mere stupidity. It is offensive stupidity.
Offensive, Marxist stupidity… question is, what can be done to stop it, if anything, or do we just have to wait until it all collapses horrifically?