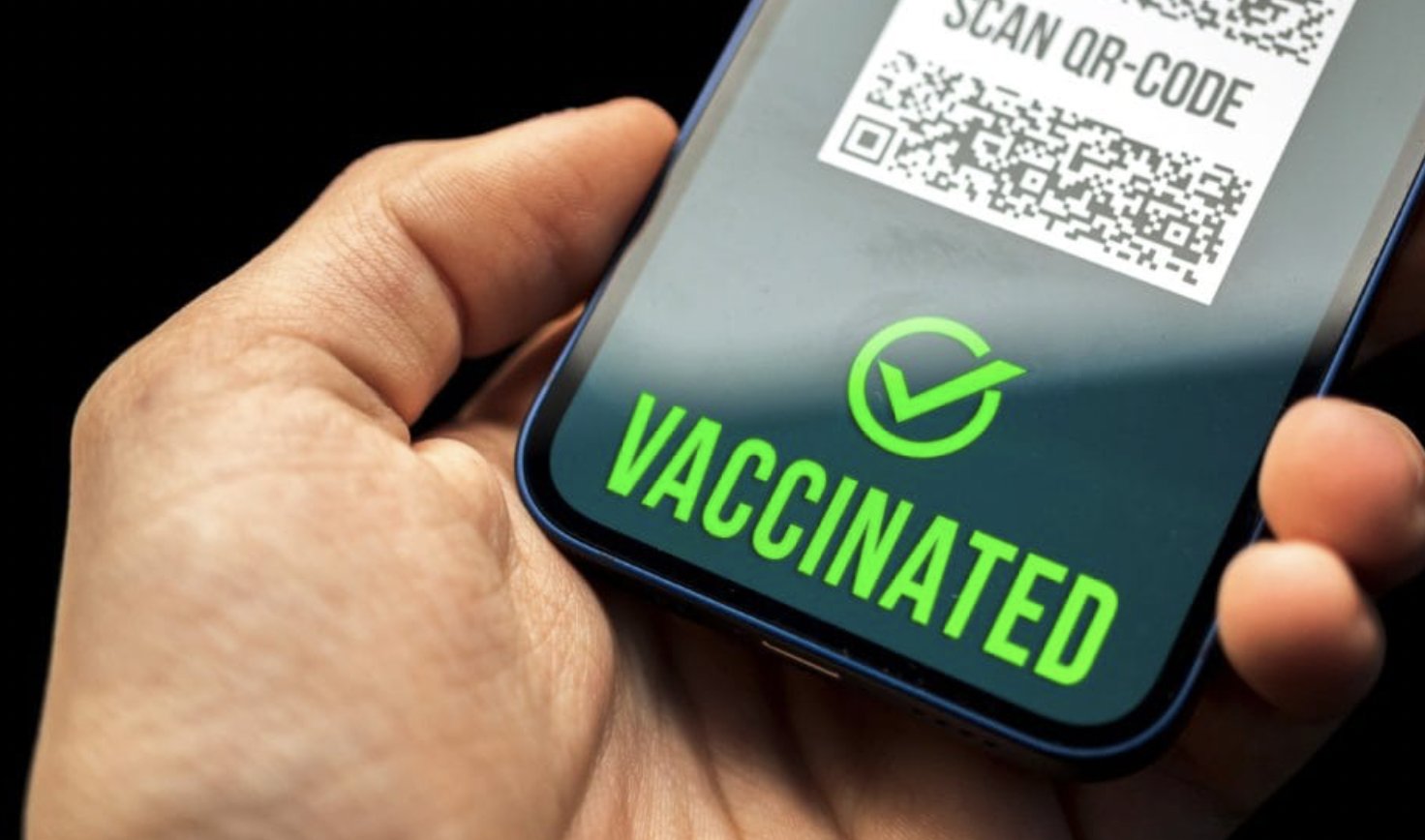
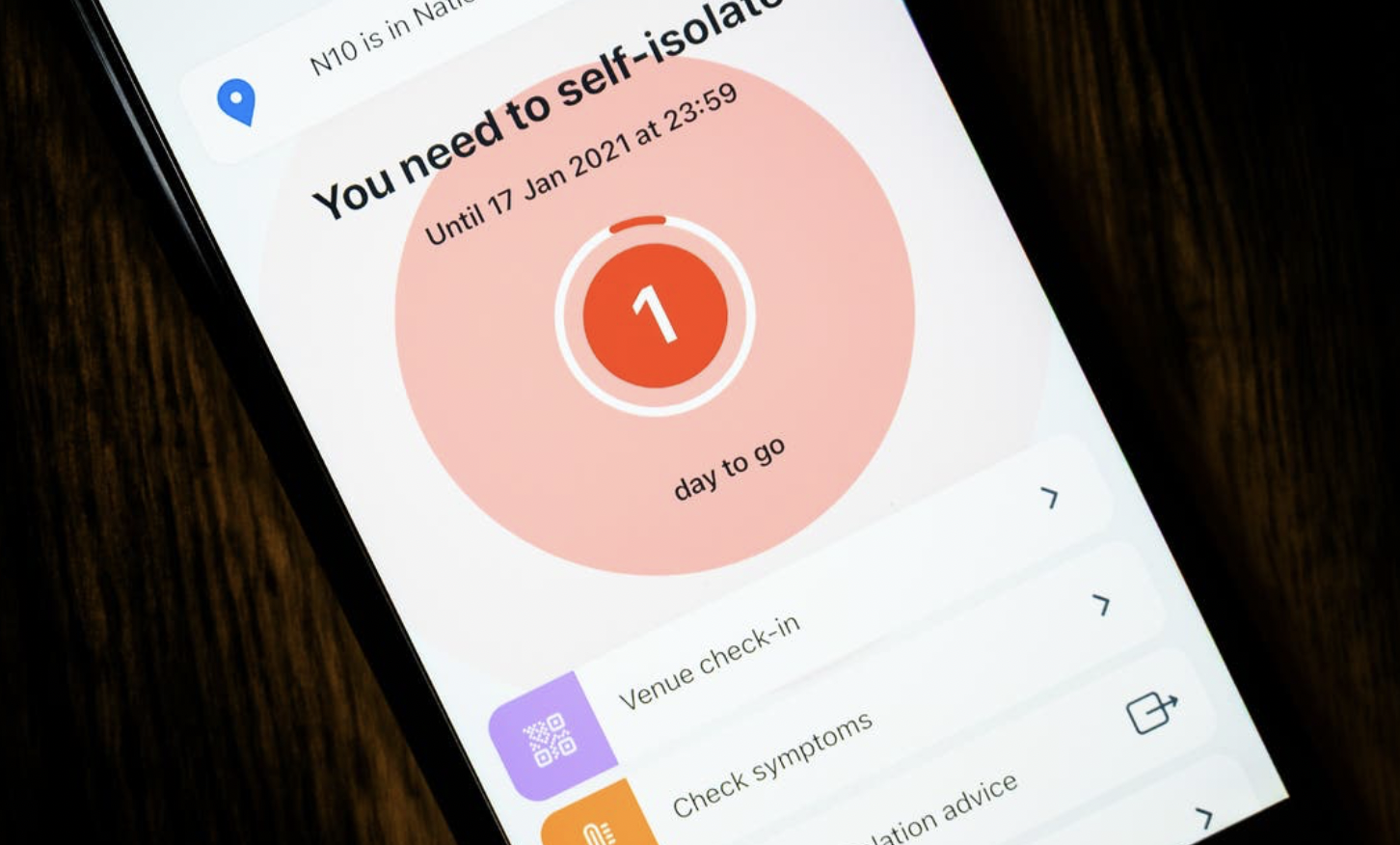
by George Santayana Informed consent is one of the cornerstones of modern medicine and the foundation of the patient/doctor relationship. The principle of informed consent is a core part of the Nuremberg Code on human research ethics and states that consent for any medical treatment must come from the patient themselves who needs to understand both the benefits and risks. Likewise, the opposite, which we might call “informed refusal”, is just as important and a patient can refuse treatment or withdraw consent at any time. The “informed’ part of informed consent can occur in a number of ways such as provision of written materials (the piece of paper you throw away when you open a packet of headache tablets) or a discussion with your doctor. Regardless, the information given to a patient needs to be accurate, balanced and cover both the benefits and risks. Consent must also be given freely and without undue influence or coercion. Of course, a clinician can express their opinion and offer advice as to what course of action a patient might take, but ultimately the decision to proceed (swallow the pill, take the test, have the operation) resides with the patient. Informed consent places the individual patient at the heart of clinical practice and given that they are the person receiving the treatment and taking any associated risks that intrinsically feels like the right thing. And so it used to be for vaccinations, where it was up to the individual whether they wished to have a specific vaccination or not. Yes, there ...