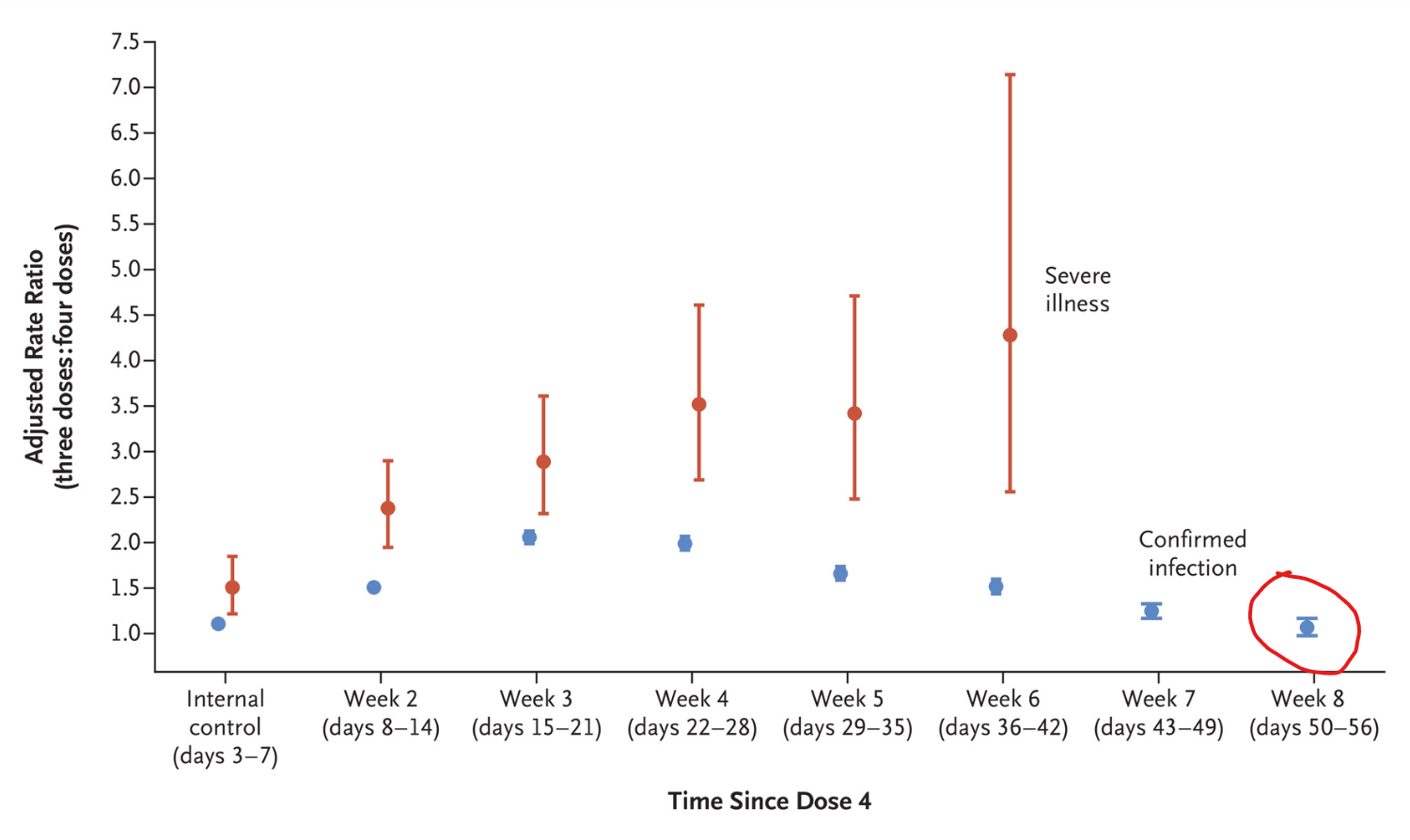
A population-wide study from Israel published in the New England Journal of Medicine has found that a fourth dose of the Pfizer Covid vaccine ceases to have any efficacy against infection within just eight weeks (see chart above). Here is the abstract.
BACKGROUND
On January 2nd 2022, Israel began administering a fourth dose of BNT162b2 [Pfizer] vaccine to persons 60 years of age or older. Data are needed regarding the effect of the fourth dose on rates of confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and of severe coronavirus disease 2019 (COVID-19).
METHODS
Using the Israeli Ministry of Health database, we extracted data on 1,252,331 persons who were 60 years of age or older and eligible for the fourth dose during a period in which the B.1.1.529 (Omicron) variant of SARS-CoV-2 was predominant (January 10th through March 2nd 2022). We estimated the rate of confirmed infection and severe COVID-19 as a function of time starting at eight days after receipt of a fourth dose (four-dose groups) as compared with that among persons who had received only three doses (three-dose group) and among persons who had received a fourth dose three to seven days earlier (internal control group). For the estimation of rates, we used quasi-Poisson regression with adjustment for age, sex, demographic group, and calendar day.
RESULTS
The number of cases of severe COVID-19 per 100,000 person-days (unadjusted rate) was 1.5 in the aggregated four-dose groups, 3.9 in the three-dose group, and 4.2 in the internal control group. In the quasi-Poisson analysis, the adjusted rate of severe COVID-19 in the fourth week after receipt of the fourth dose was lower than that in the three-dose group by a factor of 3.5 (95% confidence interval [CI], 2.7 to 4.6) and was lower than that in the internal control group by a factor of 2.3 (95% CI, 1.7 to 3.3). Protection against severe illness did not wane during the six weeks after receipt of the fourth dose. The number of cases of confirmed infection per 100,000 person-days (unadjusted rate) was 177 in the aggregated four-dose groups, 361 in the three-dose group, and 388 in the internal control group. In the quasi-Poisson analysis, the adjusted rate of confirmed infection in the fourth week after receipt of the fourth dose was lower than that in the three-dose group by a factor of 2.0 (95% CI, 1.9 to 2.1) and was lower than that in the internal control group by a factor of 1.8 (95% CI, 1.7 to 1.9). However, this protection waned in later weeks.
CONCLUSIONS
Rates of confirmed SARS-CoV-2 infection and severe COVID-19 were lower after a fourth dose of BNT162b2 vaccine than after only three doses. Protection against confirmed infection appeared short-lived, whereas protection against severe illness did not wane during the study period.
While protection against severe disease did not appear to decrease during the six week follow-up, note that the fact that severe Covid illness was occurring in the triple-dosed at over three times the rate of the quadruple-dosed suggests that the protection of three doses against severe illness had already significantly declined. This is confirmed by other studies (e.g. here and here) and noted by the authors themselves.
Read the study here. My write-up of an earlier version can be found here.













To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
The head of Pfizer would say that this is why we should be injected every month, just to be sure, eh?
Don’t give the bu99ers ideas!
We will soon be at the stage where daily injections are needed. Closely followed by an Intravenous backpack…
I’m guessing that the medical establishment are trying very hard to divert attention from this. Do they claim that the pandemic is over now?
No, the state of emergency is now permanent.
In their wet dreams! So how do they think are they going to bring in the next “State of Emergency” when all around no one is giving a shiny sh*te about the “current” one (apart from Specsavers!) Even the normies are talking about the plandemic in the past tense now! No one is talking about the “war” and it’s slowly dawning on many that the climate agenda will/is already having serious effect in their backyard, as they watch their money vapourise every month.
I remember in March 2020 when they first aired the idea of lockdowns. Everyone said they’d never do that here and if they tried it no one would comply. Almost everyone did.
Then there were the rushed injections. Everyone said no one younger than 80 would be so foolish to get themselves injected. Almost everyone I know did, and got boosters. Some have had their children jabbed.
Same for masks.
What the pandemic response has taught us is a sizeable part of the population have no real interest in personal freedom. They are easily manipulated. They will do as they are told.
I have no idea what will happen. But the last two years is one of the most successful on record for bureaucrats and technocrats. They got much of what they wanted. They will not give up. Doesn’t mean they will succeed, but more is coming.
The sheep will follow whatever is ordered and endorsed by the BBC.
Luckily for us they are a reliable source of information
He got out just in time – he must have known what was coming.
I’m still amazed that none of the comments or articles I’ve seen regarding Biden’s ‘anti-disinformation’ campaign haven’t taken the opportunity to point out that both he and Wallensky were happy to trot out the shtick about covid vaccines preventing both infection and onward transmission.
That’s my experience.
Try to tell them the BBC might be being economical with the actualite and they just won’t have it.
Ye..it is called “Johnson’s Personal Rule” as ‘Lord Vax Protector’
Johnson controls very little. It is the bureaucratic state that runs the show.
A year ago the government told the civil service to remove their staff training modules on “Unconscious Bias”, an element of their diversity training that has no basis in reality or any scientific evidence to back it up. They refused. It is still being taught to civil servants.
A minor thing but indicative of how the country is actually run. Johnson et al are actors on a stage.
I think Johnson’s roll as the clown mouthpiece is now obvious in everything he says and even the manner in which he says it.
Are we in fact now run by the CIA with daily instruction bulletins?
He loves the power the show and presumably the money.
A year ago the government told the civil service to remove their staff training modules on “Unconscious Bias”, an element of their diversity training that has no basis in reality or any scientific evidence to back it up. They refused. It is still being taught to civil servants.
Not very effective when it comes to accepting diversity of opinion, is it?
You will no doubt agree diversity training is not about diversity as such, but about narrowing the range of topics open for discussion. Diversity as taught is about racial differences, which are visible. It is an attempt to push the underlying belief we are all the same despite all the evidence to the contrary.
You will no doubt agree diversity training is not about diversity as such, but about narrowing the range of topics open for discussion.
I do indeed. We’re looking at tokenism: at the presentation of an outward appearance of the acceptance of others, at the very time that our freedom to have different and diverse points of view is under unprecedented attack.
I differ with your last sentence, however: I think it’s an attempt to make us all the same. Yes, we look different; but we all think the same way, don’t we?
And if you disagree? Let people look at what happens to scientists and doctors who don’t think as they are told, who divert from the mainstream: if they can find them. Devi Sridhar good; Sunetra Gupta bad. What a farce.
When you finally accept that these injections are NOT vaccinations (in the true sense of the word) you quickly realise why they lack any effectiveness against infection. They are experimental gene therapies, even the manufacturers accept that as fact, yet DS keeps plugging the vaccine line? Unfortunately Toby and his mates swapped sides when it became clear his chum (Doris) might fall.
What is the true sense of the word “vaccine”?
Since the official definition was changed (a few years ago), the word can mean almost anything.
I subscribe to original meaning – that the virus itself, in a weakened or deactivated form, is introduced into your person. Then the magic – your body has time to learn how to recognise it, so it can mount a quick and effective response if it does meet the real, angry, wild version in the future. You should have little to no symptoms and will therefore not get ill, die or pass it on to anyone else.
Jenner figured this out ages ago – the milk maid saying she knew she wouldn’t get smallpox because she had already had cowpox (the viruses are similar enough for the immune system’s response to cowpox to work for smallpox, or so I understand it).
The mRNA crap, in a nutshell, is Pfizer et al deciding what your body should be trained to recognise, and forcing your immune system to be very selective in what it is trained to recognise. This is a problem with coronaviruses which mutate so frequently that by the time Pfizer et al have made their gunk, it’s already out of date and your body is incapable of recognising the new variant. So-called Original Antigenic Sin.
Yes, with vaccination, the immune system is trained to recognize a whole range of epitopes – the whole pathogen.
You can read here how the definitions of ‘pandemic’, ‘vaccine’ and ‘herd immunity’ have all been changed.
https://undercurrents723949620.wordpress.com/2021/03/22/the-definition-of-pandemic-has-been-altered/
I subscribe to original meaning – that the virus itself, in a weakened or deactivated form, is introduced into your person.
You are welcome to restrict the meaning of vaccine to anything you like but it is increasingly impractical.
I had a stem cell transplant a year ago. A stem cell transplant wipes out most of the immunity you gained over your lifetime so you repeat many of your childhood ????s I will call them prophylactic injections to avoid prejudging the issue . So now I am taking a course of eight different prophylactic injections to protect me from various diseases – it turns out none of them are based on live or dead pathogens. Across the paper describing the course calls it a vaccination schedule, the medical staff all refer to my vaccinations, whenever I discuss them in conversation I call them vaccinations. Do you think we should call them something else? If so, what?
I think the coronavirus ‘vaccines’ fail on any definition of the word and since they don’t provide immunity should not bear that name. It’s a well documented fact that ‘vaccines’ have to undergo far less testing and oversight than other drugs in the US and can be marketed as a public good; perfect money making vehicles then for the likes of Gates and other shysters. There may well be examples of vaccines that are a benefit to the wider good. But these will forever now be tainted by association with these fraudulent, toxic and misnamed products.
Well, they’re definably ‘injections’, so why not go with that?
But, if you’re happy to regard them as vaccines despite the word’s etymology, go for it.
The word vaccine was redefined because people were familiar with it and proved to be more accepting of something that didn’t sound strange or new, despite the mRNA stuff being both.
You said it yourself. They are prophylactic injections or just say injections
I suspect a lot of people are going to react with incomprehension followed by “oh, you mean the vaccines” but happy to use that phrase here. Let’s see how many others stick to the same discipline.
Something you take that means you are immune from an infectious disease – you won’t get ill from said disease nor will you pass it on. Effectiveness close to 100%. That’s what most people understand by the word and that’s the basis on which the covid “vaccine” was pushed to the general public. Possibly some of the politicians thought that’s what it was, but people like Whitty, Vallance and Van Tam knew very well it was nothing like that, because they are not stupid and the designers and makers of the “vaccines” never claimed that’s what they did.
Something you take that means you are immune from an infectious disease – you won’t get ill from said disease nor will you pass it on. Effectiveness close to 100%. That’s what most people understand by the word …
That is, of course, different from Marcus Aurelius’s definition above. Something can make you immune without being the pathogen itself. However, it also rules out a lot of things what we commonly called vaccines – most obviously influenza injections which are far from 100% but also, for example, mumps and HPV vaccines which are both less than 90% effective. I have the same question as I had for Marcus Aurelius. What word should we use for prophylactic injections that provide some immunity against a pathogen but are not 100%?
I didn’t say other vaccines were close to 100% effective, but that is what people understand by the term vaccine. The flu “vaccines” are indeed a grey area, though they are tweaked annually because flu mutates, whereas none of the covid “vaccines” have been tweaked.
I don’t personally think it’s especially important to include in the definition anything referring to what’s in the thing, though clearly there is a debate to be had regarding the mechanism of a “vaccine” and approaches likely to give the best chance of long lasting, safe, effectiveness.
but that is what people understand by the term vaccine.
I am not convinced. If that were true then the phrase “60% effective vaccine” would be a contradiction in terms to most people – but I am pretty sure most people would have no problem with the phrase. Anyway it is all a bit arbitrary. All I am saying is that the Covid “vaccines” don’t differ hugely from other interventions that are commonly called vaccines in either effectiveness or in only presenting part of the pathogen to the immune system. They are unique in getting your own cells to produce that protein via mRNA (as opposed to DNA vaccines).
The influenza ‘jabs’ have always been called that, they’ve never been calked the “flu vaccine’
Are you sure? I find masses of references to influenza vaccines on the internet (try it). Every year the NHS has a flu vaccine programme not a flu jab programme.
How about: cash cowpox.
A product that gives immunity to a specific disease.
Immunity means you do not contract the disease caused by the pathogen against which you have been vaccinated if you are infected by it.
Any product that does not do this is not a vaccine.
The question is how much immunity for it to count as a vaccine? The Covid “vaccines” do provide some immunity for some time against some variants. Many things we currently call vaccines are less than 100% effective and decline over time.
My understanding of a true vaccine is something which inoculates you against developing the disease or illness which the vaccine is supposed to prevent you from getting
I guess the key to this is whether prevent means 100% forever or just to some extent for some time. The first excludes many things we currently call vaccines, the second includes all the current “prophylactic inoculations” for Covid available in the UK.
Perhaps the term “experimental gene therapies” is not frightening enough?
In RFK Jnr’s important book, he cites (p. 129) a 1977 study which used to be “required reading in almost all American medical schools”.
It found that the enormous decline in fatalities resulting from infectious diseases (74% in the first half of the 20th century) was overwhelmingly due to improved nutrition and sanitation.
It was also predicted that “vaccinologists” would hijack this success and deploy it “to claim for themselves, and particularly for vaccines, a revered and sanctified – and scientically undeserving – prestige beyond criticism, questioning, or debate”.
That’s exactly what happened. It’s why so many were eager to embrace a “vaccine” (the equivalent of holy water) that would save us from an illness of which they were unreasonably terrified, or from unbearable lockdowns (much more dangerous to our health).
Those here who have strenuously objected to the use of the term “vaccines” are right. They understand that “vaccines” and “vaccinations” are firmly associated, for the majority, with salvation.
The term “jabs” has become too cute (“just a little jab”). Perhaps they might better be referred to as “the Covid injections”.
It appears that in spite of its being constantly out of print, hardly anyone has read or heard of RFKJ’s excellent take down of Fauci and his whole gang.
Pity really, we might get better informed comments. Clearly no-one in Parliament or the MSM dares to admit having even heard of it.
I believe it’s now sold over a million copies, but that would be globally.
Books will eventually be written about this book. The Real Anthony Fauci is scrupulously researched, absolutely contemporary and a devastating critique of an important public figure and his associates: by an author who has already written best-sellers and has one of the most famous names in the world.
Bookshops which would make a fortune from it don’t sell it. The media does not review it, even to damn it. This is the book they really don’t want you to read.
I read the kindle version last year and have just ordered the hardback book
Arguably no longer experimental… supposedly the ‘magic bullet’ for cancer treatment, they have been around for years with underwhelming results.
Perhaps failed gene therapies is a better name, and failure as a ‘vaccine’ can be added to the list.
All part of the continuing roadmap!
Yep. None of this is about healthcare.
Never was.
Viruses everywhere are rolling around laughing!
“Viruses”?Do you mean psychotic, sociopathic Billionaires?
Those are the only viruses ever to have been isolated aren’t they? Certainly they’re my definition of a virus.
Unfortunately, they have broken out of isolation and are now infecting the world with their derangement!
No known cure as yet.
Ignoring them goes a long way though!
We have to remember the purpose of the injections and the rushed concoctions was bureaucratic in nature, not medical. They serve a bureaucratic function only.
We now know there are effective treatments for those badly affected by covid or any other viral infection. Some more effective than others. We also know this route was verboten through dictats from the BMA and the government, all reinforced with propaganda about horse paste and conspiracy theories.
The most damning factoid is that India and Bangladesh issued low cost covid kits containing ivermectin, vitamin D, zinc, vitamin K and magnesium to anyone who wanted them. At the time the Indians complained the west dismissed this as a third world exercise with more than a hint of snobbery and even racism.
We should be focusing on this. We are forced to pay for medical care via taxation and our doctors and medical establishment refused to consider safe treatments. All this is overlooked in the endless speculation about vaccinations and their efficacy. Zero discussion about the suppression of the effective medical care we actually pay for.
This is what shocked me when I caught covid last August, along with my uni student daughter. No treatment offered, no help given, despite awful symptoms.
The NHS were not interested in helping or offering any treatment unless you had severe breathing difficulties.
Back then I knew there were potential treatments, and that India had given out covid kits to prevent and treat the illness very successfully. Astonishing that the sum total of help from ‘Our NHS’ was advice to ‘drink water and take paracetamol.’
My daughter was isolated in her universtity digs with no treatement and no-one to look after her due to the isolation rules, but also denied any effective treatment. They even refused her antibiotics as she was isolating. Absolutely apalling.
Those Indian kits cost $2.50 each. They gave them to households for free, one for every person with special instructions for kids.
Holland and Barratt could have produced them for maybe £5. Yet ivermectin was specifically banned for treatment.
This is what we should be focusing on.
See Dr Tess Lawrie’s excellent recorded interview with Andrew Hill, the man who produced an assessment of Ivermectin efficacy , was inclined to recommend its use …but had his mind changed at the last moment.
So much out there for MSM journalists to get their teeth into …if only we had a few.
I’ve begun to wonder lately whether we don’t pour scorn on the wrong people in the MSM.
Suppose you were a decent, hardworking journo who went to his editor with an idea for a story. Initially, the ed seems keen and tells you to get more. You do so, but when you get back to the ed, he/she’s gone suddenly cold on the idea.
Even if you went ahead and did a write-up, would it get past the ed’s ‘spike’? Presumably, an editor who doesn’t toe the proprietor’s line isn’t in the job for long. And mortgages need to be paid.
It’s positively medieval the level of healthcare we are getting now. Our local hospital sends outpatients in all other directions to other units across the county rather than treat them. I haven’t seen a consultant for over two years. My mum was supposed to attend the hospital for a consultation. In the end it became a telephone appointment. They wanted to put her on a another drug which she didn’t feel she needed. She’d done some research and asked questions he couldn’t answer/didn’t want to answer. He told her to speak to get own GP, and he quickly ended the call!
 But tell them you want the stabs and they fall.over themselves to accommodate you!
But tell them you want the stabs and they fall.over themselves to accommodate you!
It is worse!
In Medieval times at least you could ask the local Wise Woman or “witch” for a herbal remedy which often worked!
Hospitals now seem more like detention centres for “medical”experimentation rather that places where the sick are healed.
My estranged other half had it earlier this year. Throat so sore he couldn’t swallow his own saliva. NHS told him to swallow paracetamol.
Pharmacist once gave me good tip for that circumstance, crush the tablet up or open the capsule, take a teaspoon of jam and mix the crushed meds into it and then slide it down (add a bit of water to make it bit more runny)
Really? Gates is on record as saying that his sponsored ‘vaccines’ will help reduce global population by 15%. What do you think he meant by that?
I believe Gates’ rationale was the general improvement in health would mean people have fewer kids.
However, Bill Gates doesn’t run Britain, the government does. It was them who made every decision and need to be held to account.
Dom Cummings is on video record giving evidence to HOC Committee that W Gates was advising the UK government about how to handle pandemic. Government runs Britain yes, but who was it taking its advice about how to run the country on a KEY issue from?
Yes, indeed. This IS what the likes of Mark Steyn should be focussing on. The fact that government and health authorities denied people the use of effective treatments like Ivermectin and Hydroxychloroquine is tantamount to mass murder. Who took these decisions?
This short video of an exchange between Dr Tess Lawrie of the British Ivermectin Recommendation & Development Group (BIRD) and Dr Andrew Hill who had undertaken a study for WHO is enlightening as well as stomach churning.
https://bird-group.org/dr-hill-changed-his-mind-on-ivermectin/
This video is absolutely sickening and Mark Steyn should feature it on his programme
We pay for medical care via our taxation – we cannot now access the medical care we pay for via taxation so we have to pay again, to go private, to get the medical care we have already paid for via taxation, which, as you rightly point out, nobody discusses (Where are you on this issue GB News???)
“ India and Bangladesh issued low cost covid kits containing ivermectin, vitamin D, zinc, vitamin K and magnesium to anyone who wanted them. At the time the Indians complained the west dismissed this as a third world exercise with more than a hint of snobbery and even racism.”
The big question in this debate is – why did India and Bangladesh go down this route? Are they not in on the WEF programming and diktats? Or did they refuse? Or is it just taken as a given that they will go along with the programme?
The FDA was actively considering banning the sale of the supplement NAC because it was an effective covid treatment (I’m not sure if they went through with this but it was definitely on the agenda at one stage)
Pfizer showed this on their Q1 earnings call. Of course their interpretation stopped at 4 weeks. It was farcical.
From the transcript:
Evan David Seigerman – BMO Capital Markets Equity Research – MD & Senior BioPharma Research Analyst
So I have 2 on COVID: one, when we think about the evolution of the booster market, how do you see this going? We’ve seen some data suggesting that we’re kind of walking blindly into recommending boosters every so often. What’s the ultimate goal of vaccination? Is it to prevent mild, symptomatic disease? Or is it really just to prevent the severe disease and overloading at the hospitals?
And then my second question is when we think about the evolution of the commercial model for PAXLOVID, can you talk to that? When do you expect to maybe file an NDA and transition away, at least in the United States, from kind of government contracting to more traditional commercial model?
Albert Bourla – Pfizer Inc. – Chairman of the Board & CEO
Yes. Thank you for your questions. Let me see if I can answer and then I will ask our scientists if they have something to add. On the evolution of booster, I think all authorities, they are not moving blindly. We are moving based on data. And the reason why they recommend what they recommend is because data are supporting according to their opinion. I believe that right now, the effort it is to be able to stay ahead of the virus and the virus mutates and the most serious mutation that we have seen was the Omicron, one, because there was the one that was able to evade the new protection of us.
Until then, I think the vaccines were offering very, very good protection against disease and, of course, an excellent protection against hospitalization and death. With Omicron, we saw that while we keep a very good protection against hospitalization and deaths, the protection against the disease is going down. So we have seen that with our data that a fourth dose of the current vaccine protects significantly folds, many folds, the patients from either hospitalizations or death and, of course, also protects the infections but not to the degree that it used to be.
Everybody is working also for new vaccines that will be able to protect better against Omicron while maintaining the same protection against the wild types. We are very advanced with our studies. And we are waiting to hear from FDA what basic combinations they would recommend, what they would like to see at the EMA, and we will be ready day 1 with our vaccines, both in terms of filing and both in terms of manufacturing. As regards PAXLOVID, right now, we are completing the studies and I will ask Mikael to comment when we think that we can file for a full NDA.
Bullshit non-answer. Buck passing to regulators, acting like they are handed goals by FDA, when in reality it is a lengthy negotiation.
May I suggest reading ‘Dissolving Illusions – Disease, Vaccines, and the Forgotten History’ by Suzanne Humphries and Roman Bystrianyk.
It is indispensable during these deceitful times and will give a clear insight into the mind-set of those implementing Rockefeller-based ‘medical science’; such as non-stop ‘vaccines’ for something the healthy human body does not need…
Have you read Virus Mania by Torsten Engelbrecht, Claus Koehnlein and Samantha Bailey?
I would say that this book takes Dissolving Illusions to the next level
Agreed.
I’ve had an aspirin which provided longer resistance to a headache than these dangerous jabs.
OK, not really, but that’s the way these jabs are going. A vaccine provides lifetime, or long-term protection from illness. Not 8 weeks and decreasing …..
The injections were a test for compliance only. Their goal was not healthcare.
Armed with that data the government will now amend it’s approach accordingly.
Is this Pfizers solution –
New Frosties now fortified with covid19 vax, they’re great.
the fact that severe Covid illness was occurring in the triple-dosed at over three times the rate of the quadruple-dosed suggests that the protection of three doses against severe illness had already significantly declined.
Can someone unpack that argument for me? I don’t get the logic.
Anyhow glad to see four doses provides a substantial additional protection against severe illness which is slow to decline as I had my fourth does in March.
Logic seems clear to me. What part don’t you get?
He seems to make a career out of not getting it.
and that seems to be the whole point of his questions – to derail the debate on an otherwise decent discussion
If the triple-dosed had the same protection against severe illness immediately after the third dose as the quadruple-dosed currently have against severe illness, immediately after the fourth dose, then if currently severe illness is running at three times the rate in the triple-dosed as in the quadruple-dosed, then the protection of three doses alone must have already declined by a factor of three.
Yes – but it is also possible that the triple dose offered good protection against severe disease which did not decline and the fourth dose made it three times better. The summary does not give the rates for severe illness but there is nothing in the paper that excludes the rate for four dosers being x, for three dosers 3x (with no decline) and for the unvaccinated being 20x.
None of the uk data supports that scenario. Notice the controls are 3 dose and 4 dose but early after dose. Where is the control against truly unvaxxed with no prior infection or unvax with natural infection. This is why they were so desperate to remove the control group (althought this study seems to have done it anyway), then they can just compare to the previous waning dose and say everyone needs a booster. Lots of studies show natural infection provides better protection. Plus rockoman has posted the link that discusses the lack of all cause mortality data in the study. Its no good reducing someones susceptibility to one thing if it makes you more susceptible to other things. Consistently studies have shown all cause mortality is at best no better in vaxxed and considerably worse in some ages. Then add in the vax deaths which have been hid in the data from the very start.
I would encourage you to use the search term ‘UKHSA’ on this website. There you will find a great deal of information showing that after an apparent positive effect in the short term efficacy rapidly goes negative.
Joel Smalley’s substack also has a great deal of info and analysis in this regard. Perhaps start here:
https://metatron.substack.com/p/covid-requiem-aeternam?s=r
Article and video.
“There is a discernible reduction in the rate of COVID deaths in just 38 out of the 202 countries studied (19%). Therefore, in the vast majority of countries, both the rate and the number of COVID deaths after vaccination programs is higher than before.”
See Senator Robert f Kennedy book for further info on this.
It’s scary and illuminating at the same time
The study only made a comparison between 3 and 4 jabs. It said nothing about absolute levels. Unvaccinated didn’t figure in the study so mentioning it is pointless (other than politically).
The fact that they’re studiously ignoring how purebloods are doing is itself telling. Our own Ministries of Health Truth are also burying those numbers so as not to confuse people with facts and information.
If the data from Israel is correct, then your 4th dose should be wearing off round about now.
Better get back in the queue.
As it happens, I qualify for a fifth three months after the fourth.
sorry to disillusion you but the effectiveness of the 4th jab is dead in the water after 2 months says Israel study. You might have a few days protection left or none at all depending on when you had it.
Maybe – I as it happens I took part in a research project on antibodies and I know that a few days ago my G antibodies were very high (I never had Covid)
Of course it doesn’t, buy then it tops up those pathogenic spike proteins in the vax that carry on wrecking your immune system and developing VAIDS – isn’t that what it’s really all about?
Basically, to fall for the whole ‘vaccine thing’ you had to believe that scientists had been working without success to find a vaccine against coronaviruses, including Sars-Cov-2, for decades without success, but then, within well under a year. not one but six different products were on the market, all claiming to be tested, safe and effective vaccines against Sars-Cov-2, when the whole process, of necessity, must take much longer, even if successful.
We know that Moderna ( Mode Rna?) were working on Gene Therapies for years without having one considered as safe enough for trial use. It was all lined up to match the’ gain of function’ manufactured Virus ( see Peter Daszak).
The truth is out there for all to find.
Self deception on a massive scale. My GP included. In 2020 I asked her when/if there would be a Covid jab. She shook her head gravely and said with her German accent “ooh no not for 10 years” because of safety concerns, she even doubted there would be a jab at all. Yet she fell for the whole thing hook line and sinker later urging me to have it.
https://galileoisback.substack.com/p/9e4ea0e3-500a-412e-beb9-426c9a2fb587?s=r
More problems with the official data about the fourth dose in Israel.
Always look for what they havent told you and why, not what they have.
None of the jabs ever worked, the reason that deaths dropped is that the weakest fell first.
Could ADE be about to flatten the vaccinated ?
https://thehighwire.com/videos/the-vanden-bossche-warning/
Well worth a watch, stick with it as it gets interesting!
The number of cases of severe Covid-19 per 100,000 person-days (unadjusted rate) was 1.5 in the aggregated four-dose groups, 3.9 in the three-dose group, and 4.2 in the internal control group.
that’s about the same rate of severe covid cases as UK road deaths per 100k.
you’ve got more chance of doing in your car than of covid based on this bullshit .
I note the word ‘estimation’.
In a population two years into a circulating virus, most vaccinated at varying times with different doses, different ages and health conditions, many or most exposed naturally to the pathogen, any observational studies are meaningless.
The fact that single/double/triple vaccinated people are ‘severe’ cases and dying, tells us the pseudo-vaccines aren’t worth spit, so let’s stop splitting epidemiological hairs and admit it.
Isn’t this old news?
I seem to remember reading that 4th-shot efficacy lasted only 8 weeks some time ago.
(Or perhaps I’m just remembering my nightmares again.)
I know what you mean.
It’s getting to have that Nineteen Eighty-Four feeling.
All the ‘news’ seems to have happened before.
i’m sure the 5th jab will do better, if not, i am very very sure the 6th 7th and 8th would do the trick, but just to be sure, better take 9th 10th and 11th jab, but if in doubt, 12th and 13th are there anyway.
666 would surely do the trick.
Obviously we must push on with the fifth jab to fill in the gap.
Perhaps every gp should have told this to everyone of their patients. The nhs, it’s medical providers are one of life’s bigger disappointments.
you have to
lower‘adjust’ your expectations I am afraid when it comes to the NHS – then you won’t be so disappointed.In this complex article from Israel they found new mutation in omicron and the conclusion is that even the 3rd dose will be ineffective for omicron
https://www.biorxiv.org/content/10.1101/2022.04.26.489630v1.full.pdf
P681 mutations within the polybasic motif of spike dictate fusogenicity and syncytia formation of SARS CoV-2 variants
“We conclude that over time, the efficiency of the third dose of the Pfizer vaccine against SARS CoV-2 is waned, and cannot neutralize Omicron”