There follows a guest post by Dr Ros Jones, a retired Consultant Paediatrician and member of HART.
We have heard a lot in the last few weeks about Yellow Card reports for any adverse effects of vaccination, so I shall seek here to give a little background to the system and where it can work well but where it can seriously fall short.
The Yellow Card system was introduced in 1964 following the thalidomide disaster as a way of formalising the reporting of adverse effects, especially for new drugs. Tear-out cards, printed on yellow paper, were inserted at the back of the British National Formulary (BNF), which acts as the bible for UK prescribing. This book, updated twice yearly, was given to every practising doctor. Any doctor or pharmacist seeing a patient with unexpected symptoms relating to a prescribed medicine could quickly complete one of the cards and send it to the regulatory authority. But already you can spot the problem here – the system depends on the health care professional recognising that the symptom might be related to a particular drug, so if the connection is not made then neither is the report. Take for example a busy orthopaedic SHO treating an elderly lady with a fractured hip. Will they think to report this as an adverse reaction to her blood pressure tablets? This matters. Studies show up to one third of hospital admissions are due to iatrogenic causes i.e., drug side-effects. Nowadays, the BNF is an online book and the Yellow Card system is also online, so perhaps even more ‘out of sight, out of mind’, especially if the ward is really busy at the time. If you ask colleagues whether vaccine adverse outcomes have been reported to MHRA, they often reply: “I’m not convinced it was the cause, it could have been due to anything.” But physicians are not responsible for deciding whether a clinical event was caused by a drug or was coincidental – that is the role of the MHRA.
All new drugs and vaccines are subject to trials, starting with animal trials usually involving a number of different species, then building gradually through small pilot studies on humans to establish dosage regimes (for example) and short term safety, before rolling out to large scale trials looking for both efficacy and longer term safety. In such trials, all adverse outcomes will be reported, with the control group acting as the base-line for any symptoms against which the new drug is compared. The system works well for reasonably common side-effects and here the size of the trials is important. You will see in drug information leaflets side effects listed as “very common: affecting greater than 1 in 10 people”, through to “very rare: affecting less than 1 in 10,000”. Generally speaking, “very rare” side effects are only listed if severe. Many drug and vaccine trials are only large enough to detect “uncommon” side effects and for any new drug it is only through post-marketing surveillance that rarer side-effects can be discovered. New drugs are marked in the BNF with a black triangle for two years, to remind doctors to complete yellow card reports. In addition, most drug trials will exclude certain groups – for example children, pregnant women and people with risk factors such as kidney and liver disease, so safety for these groups is very much dependent on animal studies or assumptions from other similar drugs. Species difference in adverse effects may occur too, so damage to the developing foetus may only be seen after a drug starts being used by humans. Certain age groups that are under-represented in trials, such as the very elderly, may also be at greater risk. If post-marketing surveillance reveals an unexpected problem then the drug licence may be withdrawn or modified (e.g. limited to certain age groups, as with the AstraZeneca vaccine).
Where an adverse event comprises something very rare, it may be obvious to the attending physician (or indeed the regulator) that this is a potential drug effect. So for example, thalidomide caused a limb-shortening birth defect, phocomelia. This was totally new to the obstetricians and midwives of the day, so it was quite quickly realised that this was a teratogenic effect. But because of the time lag between drug ingestion in early pregnancy and the discovery of the harm, despite withdrawing the drug, over 10,000 babies were affected across Europe. Turning to the recent concerns raised with SARS-CoV-2 vaccines, cerebral venous thromboses (CVT) are rare. A general physician might only see one case in several years, so when a number were reported, it soon became apparent that they might be linked to the vaccine. But if the side effect is a significant increase in a common condition, it may be much less obvious. An elderly patient admitted with a stroke would not ring alarm bells but if suddenly a lot more people were dying in a two-week period after receiving a new treatment, then this could be highly relevant. Another concern for the vaccines is that they have been granted a temporary licence ahead of the long-term safety reports. Over time, many of those volunteers in the control arm of the trials will have received a vaccination and the power of the randomised controlled trial in terms of assessing safety will be greatly reduced.
So what does a prescribing doctor make of all this? We are taught, “first do no harm”. This does not mean you cannot prescribe a drug with known side-effects (that would rule out most effective treatments!), but it does mean that you have a duty of care to consider the patient in front of you and make a judgement on the balance of risk, i.e., do the likely benefits of this drug outweigh the potential harms? With vaccines, this is made more complicated by the fact that the person in front of you is not ill. Thus, you are weighing up the benefit of preventing severe disease or death from a condition which they may or may not catch (indeed against which they might already have immunity) versus a risk of a significant adverse reaction. Clearly this calculation will be different according to susceptibility to the disease in question, so for SARS-CoV-2 vaccines, a safety threshold for a healthy young adult let alone a child would be set much higher than for a 75 year-old with diabetes.
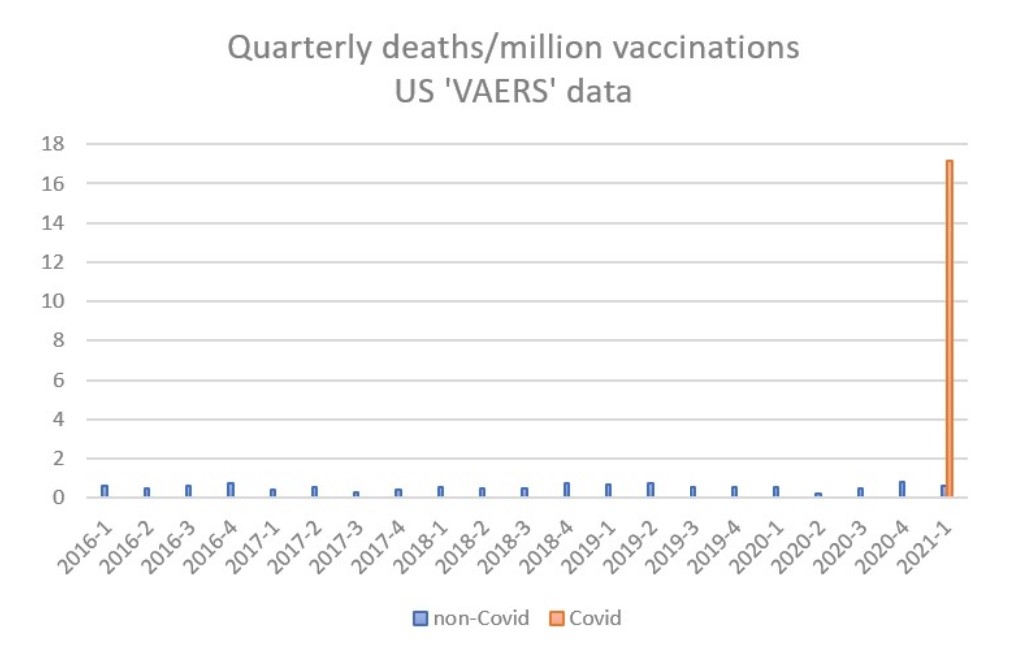
What of the Yellow Card system – is it fit for purpose? I would argue that in the current situation it is not. The MHRA does not provide raw data for scrutiny but the publicly accessible US vaccine adverse effects reporting system (VAERS) illustrates the scale of the problem. From 2016 to 2020, non-Covid vaccines generated an average of 13 reported deaths per month, which, taking account of the number of vaccinations, equates to 0.56 deaths per million doses. In the first quarter of 2021, a total of 2,103 deaths have been reported following Covid vaccines from 130 million doses, giving a rate 16.2 per million doses, i.e., a thirty-fold increase on the background rate. This extraordinarily high rate has been apparent from the first month of Covid vaccine administration, and is not declining.
It is also notable that reports of CVT in the UK increased significantly after several cases had been reported in the press, thus raising awareness amongst medical staff and leading to a change in advice from regulators.
When rolling out a new drug to a whole population which uses a new and untried technology and which has been commenced under temporary licence without the benefit of long-term safety data, it is vital that any post-marketing surveillance is rigorous. As a minimum, every GP or hospital visit should record vaccination status; everyone receiving a vaccine should be given a pre-paid, pre-addressed card with a tick box for any side effects, to be returned to the MHRA 30 days after vaccination. Only then can even short-term safety be assured.











To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
To emphasize once again – the miniscule benefits are the other side of the equation at the current low rate of incidence of the virus.
Put the benefits and harms together, and any rationale for the ‘vaccines’ disappears. The emergency licensing should be withdrawn immediately.
To emphasize once again – The main driver of current low rate of incidence of the virus is vaccine.We are still in the season where the virus can take off, this time last year,Boris was in hospital with covid19.This time last year we having ~ 1,000 covid death a day.9th April 2020, 981 covid deaths.10th April, 980 covid deaths.Outdoor hospitality was closed until July 3th last year.
We got it easy this year. mostly down to vaccination and the warmer spring.
Warmer Spring???
This spring has been much much colder than last year. This time last year we were out walking in shorts & t-shirts, this week we wore winter coats to sit outside the pub.
I’m afraid fonzi doesn’t do reality. He may have even been rejected by 77th Brigade – and is still trying desperately to please!
you are pon course, with Boris and the other anti-vaxxers for another longer harder lockdown. you deserve it.
Yes Boris is such an anti-vaxxer isn’t he? Are you sure you are in this country?
I’m afraid RickH is obvsly a government plant placed here to lengthen lockdown, by undermining vaccine.
Better than undermining civilisation.
Oh dear, oh dear Fon. Do you not understand all the data that has been presented here over many months?
Lockdowns don’t work. Never have, never will.
The ‘vaccines’ – why would anyone who is fit, healthy and under 75 want a vaccine(here I use the term loosely) for a disease that IF they get it they will make a full recovery or have such a mild illness that only a few days off work are required.
To call people ‘anti-vaxers’ is a total insult. I myself have had Typhoid, hepatitis A and B, smallpox and tetanus. I do not wish to have a modified RNA vaccine for something that i may never get.
You might be righ it’s perhaps too soon to drop out guard, on the other hand if the weather is not doing the heavy lifting, that leaves lockdown or vaccine. RichkH and Boris prefer lockdown, mugs, both of them.
Or more likely increasing immunity in the population (even if we haven’t quite reached herd immunity) in large part due to prior infection. There was never a binary choice between vaccines or lockdown, consider Sweden etc. and the GBD as other options.
I prefer to live my life and make my own choices. I know 3 people who think they had covid before testing was available, and no one since. The only deaths I know about personally were in a care home where they regularly lose half their residents over winter. Difference this time was they all died within a fortnight of being jabbed, and that after almost a year managing to stay covid free. So call me an anti vaxxer if you like, if you take it that’s your choice, I won’t be bullied.
Probably writing that from a secure PLA facility in Beijing.
Yes, needs some extra tuition in EFL for sure.
are you with grammarly, in any case , please do one.
What type of bribe would it take for the first minister of Wales ,Scotland, the north of Ireland and the Prime minister of the United kingdom, as well as all their various ministers and advisors in all capacities and for the equivalents in all G7+ nations to accept a dishonest/secret lockdown plot?
Some of the ideas put forward here are crackpot ideas. There is an honest case to be made that hard lockdowns alone are too brutal to do more good than harm, but it’s a form of insanity to conclude that vaccines are futile or part of an even larger international plot.I Peerhaps it’s childish to thank that way.So I honestly ask what size of bribe would it take to make the fears real? Please think it through And let me know,
it might be waking up time, folks…
This time every day for the last, well, years, we lost 1500 people a day to something. This is not news.
Aside from the days when we were losing an additional 1000 per day. Want low incidence? Then one has to take the medicine. That medicine is either no interaction or vaccination. Society has chosen the latter after a year of the former. Sadly there is no such thing as an entirely safe medicine.
Nice article.
Anti Vaxxers are hard of statistics.
To end this we need to get to herd immunity. You get there by getting the disease or getting the vaccine for the disease.
Death from the vaccine is about 1 in 45000 chance. Death from the disease is about 1 in 250 – skewed by age.
If you’re over 40 then the vaccine is a no brainier.
40? Maybe 65
I’m 50, fit and in good health, not obese, never suffer colds or flu. My chances of firstly getting the virus and then being seriously ill with it are most certainly less than my odds of having adverse reactions to the ‘vaccine’ should I take it.
The odds of having some form of adverse reaction to the Covid vaccines, that aren’t vaccines, are clearly exceedingly high. The chance of having Covid-19 on your death certificate is very low, though it increases markedly, if you happen to be already gravely ill from a non-Covid cause and are also of a vintage greater than that of the average age of death.
I thought that a year ago too. I was one of the fittest 50yo in the country. It’s taken a year and I’m nowhere near back to where I was. The odds of serious adverse events with the vaccine are much lower than serious covid with infection.
“The odds of serious adverse events with the vaccine are much lower than serious covid with infection.”
Nobody has any idea of the serious adverse consequences that might result in the long term from being injected with these experimental treatments.
I thought they were trials till 2023, who would volunteer for this?, to put more money into the most cynica lCo’s, It makes no sense to anyone with a critical facility.
The odds of serious adverse events with the vaccine are much lower than serious covid with infection.
The problem is we can’t really say that with any certainty at the moment. The Phase III trials for all the vaccines won’t be completed until 2023.
Maybe you’re like the only guy I know that’s “suffered” long Covid? Every time I’ve bumped into him, he’s had a bottle or two under his arm.
So you were one of the very few unlucky ones. But saying i should take the vaccine because you got ill is about the same as saying I should put all my money on black at a casino because you won on black. Nonsense.
Here we go again, why not tell them about your underlying conditions that you don’t consider serious. Or maybe what your day job is!
A brainless reply! That’s like saying I was ill with a heart attack, so I’ll risk chemotherapy because it might help.
I’m 67 cycle 3-5 times a week play golf twice a week (Government permitting) walk the dogs twice daily and work on the allotment. My chances of dying from Covid are more than 1/1000 (and I’ll have to contract it first, which is not easy currently). My chances of dying in a RTA are 1/240. The vaccines are experimental medicines out on emergency license. To date nearly 800 have died from side effects in this country alone and there have been over 600,000 adverse side effects. I have had colds (plenty of them!) and flu so assume gathering from various scientific papers that I have some sort of T cell immunity. I also take Vitamin D supplements, zinc and a large glass of freshly squeezed orange juice every morning. Rightly or wrongly I have decided not to have the vaccination. My body my decision. My Partner on the other hand is over 70 and more vulnerable decided (probably from bullying from her family) to have both vaccines. Her body her decision. What I also don’t like about all this is the subversive Government coercion which has probably also played a part in my decision being of bloody minded stock.
Loved your comment about dying in an RTA. i live in a country which is No 1 for road deaths – average 65/day, population same as England so at a rate of 1/250(Presuming you are in the UK) I wonder what my chances are?
Hi Judy yes in England actually. I looked up odds on Google presume you could do the same for where you live. Have a good day.
Yes the more they try to pressure me the more determined I get.
Since I changed my diet 16 years agi I haven’t had the flu and only two colds. I’m pretty sure I already had covid back in december 2019, it was obviously something strange to break through my immune system. I want to keep it just the way it is thank you.
You have to add the 1 in 250 chance of death to the odds of actually contracting the virus to begin with.
The trials of all the Covid vaccines are far from being completed and so the vaccine death figures cannot yet be known. The figures you quote are clearly poppycock.
Death from the disease was caused deliberately by coordinated actions never before taken in response to any disease: 1. Do not treat until patients are dangerously ill. 2. Do not treat with medicines and protocols known to be effective.
That’s not true. I’m 48 and the Oxford online tool puts my COVID risk of death at 1 in 60,000. And risk of hospital admission at 1 in 4,000.
So I say, it’s a no brainer not to get vaccinated. .
Age has nothing to do with it. Many 90 years olds have fought off Covid. They are generally vulnerable to all sorts of things. What matters is health terrain. If you are obese, have other serious health conditions or diseases, then you are more susceptible.
its not really age, its health. if you so ill you are likely to die in the next year then it might be worth taking the vaccine – then again why bother
It’s not worth taking the vaccine if you stagger on for a couple of months without Covid or flu, but then develop sepsis, have to have a double amputation and spend your last few months on this Earth in absolute misery. We need to get over the idea that all deaths are bad. They aren’t and not for nothing was pneumonia previously known as “the old man’s friend” (and old lady’s no doubt).
There cannot possibly be any statistics at all anywhere that would give me any reassurances about the medium and long term consequences of taking these vaccines. It just isn’t possible to compress time.
P.S Your ‘1 in 250’ isn’t even accurate as an IFR estimate.
This is not about one vaccine. Are you really naive enough to think that vaccine passporting won’t be extended to other diseases and conditions? It’s about how you create the conditions where people can be healthy and lead rewarding lives. People who think the road to good health is by vaccination for common respiratory diseases (which will simply create opportunities for more novel pathogens, possibly very deadly ones), hundreds of vaccinations per year for individuals, more Big Pharma drug treament, lockdowns, mandatory mask and intrusive test and tracing are deluded and are leading us into a dystopian nightmare.
Deaths from the vaccine are clearly much higher than 1 in 45000 because we always see deaths soar when mass vaccination programme start and they begin vaccinating the most vulnerable. It’s just governments refuse to investigate the association and the MSM also cover it up.
Aha, so here you are again spreading the same discredited nonsense about 0.4% Covid 19 deaths? It remains utter b*ll*cks no matter how many times you (and maybe Prof Ferguson from IC, where this figure doubtless has its origins) repeat it!!
Once again – FYI (and Ferguson’s, and the Govt’s):
Factoring in the chances of even developing Covid-19 at this stage in the game (as mentioned above, 1 in 500,000 according to Tim Spector who runs King’s College Zoe App) suggests that jabs for the healthy under-65s are statistically nonsensical.
For more info…
https://www.conservativewoman.co.uk/vaccine-risks-versus-rewards-what-your-gp-wont-tell-you/
I know it’s a little point, but again, the Jabz does not confer immunity.
I nominate “skewed by age” for the understatement of the year award. Of course we might add “skewed by health” but we have a healthcare establishment that doesn’t much care about health.
Except that’s not true is it, else we would still be seeing the 1918 flu sweep around the world killing 20 year olds, and the 1968.
And none of those needed either vaccinations, or draconian restrictions to stop.
Now no-one wants a repeat of the 1918, but frankly, disease is a risk of living: even if fergunson’s worst prediction came true, we would be living in 2020-21 with roughly the same risk profile of 1976, where they were quite happy to continue life as normal. And 1918 flu wasn’t apocalyptic for society, whilst lockdown is. It’s worth noting that, despite a wild variation in different levels of vaccination and NPI nowhere on earth has come close to the pre-pandemic plans death levels, and it’s not likely they would have done, and under those plans life would have continued as normal.
Humans were put on earth to flourish in a society, so the idea that no interaction is a valid medicine is abominal.
So no, my freedom isn’t conditional on either low incidence or high vaccination numbers, and the idea it is, is simply rank cowardice.
I refer you to the following articles on this site which utterly refute your dogma:
https://dailysceptic.org/2021/04/18/if-lockdowns-are-needed-why-did-more-people-die-in-us-states-which-locked-down-than-those-which-did-not/
https://dailysceptic.org/2021/04/18/sage-modelling-from-may-last-year-said-approach-recommended-in-great-barrington-declaration-was-least-bad-alternative-to-lockdown/
The overall mortality rate for those infected by Coronavirus 1984 is 0.15% – 0.2%, like a strong flu which typically has an IFR of 0.1%. Covid19 is actually less lethal in the under 70s than regular flu.
“Global infection fatality rate is 0.15‐0.20% (0.03‐0.04% in those <70 years)”
This research comes from the world’s number one Data Scientist: Prof Dr John Ioannidis – Professor of Medicine (Stanford Prevention Research), of Epidemiology and Population Health and by courtesy, of Statistics and of Biomedical Data Sciencehttps://profiles.stanford.edu/john-ioannidis
Global perspective of COVID‐19 epidemiology for a full‐cycle pandemic
https://onlinelibrary.wiley.com/doi/full/10.1111/eci.13423
There has also been substantial amounts of mislabelling of death to bump up the numbers since this whole charade started and people have had Covid19 put on their death certificates when there was no sign of any Covid19, for all sorts of different reasons, so all the evidence points to a fairly standard to strong flu season plus a lot of effort made to fraudulently get Covid19 on to death certificates even if that meant committing forgery, which is now being exposed in legal challenges like this one going through at the moment – private criminal prosecution for fraud against Hancock, Whitty, Vallance and Ferguson:
R [PUB] v Hancock & Others [2021] | Public Notice of Intended Prosecution
https://www.thebernician.net/r-pub-v-hancock-others-2021-public-notice-of-intended-prosecution/
Extremely reckless and dangerous measures taken by the government have led to an uptick in deaths, from the over subscription of ventilators to the expulsion of the elderly to care homes en masse to the ‘only one disease gets treated’ mantra of the NHS and the rollout of untested experimental vaccines to the general public, the relentless fear-led brainwashing leading to people not seeking treatment, depression and so on.
The British medical establishment has gone to the trouble of running sham trials to discredit licenced drugs in their quest to make it appear that the only possible solution to “the greatest public health crisis ever” is the experimental gene therapy being hawked like it’s the best thing since sliced bread, which it most certainly is not.
The Lancet Retracts Hydroxychloroquine Study
“June 4, 2020 – The online medical journal The Lancet has apologized to readers after retracting a study that said the anti-malarial drug hydroxychloroquine did not help to curb COVID-19 and might cause death in patients.”
https://www.webmd.com/lung/news/20200605/lancet-retracts-hydroxychloroquine-study
All this with the full knowledge that there is every chance that the experimental, approved for “Emergency Use Only” gene based vaccines will lead to an epidemic of Vaccine Enhanced DIsease or Antibody Dependent Enhancement, because every attempt to create a Coronavirus vaccine in the past led to this exact phenomenon in 100% of the animal trials, hence why they never got licenced. mRNA therapy has also never been approved before the emergency approval was granted. This is a Coronavirus mRNA gene vaccine rollout……
For Covid1984 they skipped the animal trials and went striaght to population-wide rollout, while running sham trials to discredit drugs that were lying in the shelves of every hospital in the world, which showed great promise in treating patients with severe disease. There were even reports of HCQ factories going up in smoke in the Far East which was suspicious to say the least. Readily available drugs that are already approved for use being used to treat Covid19 would render their vaccines unecessary and too high-risk and so they would never have got emergency approval, because safe treatments were already available.
Informed consent disclosure to vaccine trial subjects of risk of
COVID-19 vaccines worsening clinical disease
https://onlinelibrary.wiley.com/doi/epdf/10.1111/ijcp.13795
“Conclusions drawn from the study and clinical implications: The
specific and significant COVID-19 risk of ADE should have been and should be prominently and independently disclosed to research subjects currently in vaccine trials, as well as those being recruited for the trials and future patients after vaccine approval, in order to meet the medical ethics standard of patient comprehension for informed consent.”
18 Reasons I Won’t Be Getting a Covid Vaccine
#3: THE UGLY HISTORY OF ATTEMPTS TO MAKE CORONAVIRUS VACCINES……
https://www.deconstructingconventional.com/post/18-reason-i-won-t-be-getting-a-covid-vaccine
spot on , the cretins want the moon on a stick, rejecting vaccine = more longer harder lockdown, they seem to enjoy it and map it up like wild dogs.
Seeing a wild dog mapping a moon on a stick would be a right giggle.
Woof.
The “moon on a stick” is what we had every year before 2020.
Aren’t you the same guy who made an emotional plea for civilised debate before supposedly leaving this website around December?
The snake oils don’t prevent infection.
The snake oils don’t prevent transmission.
The snake oils are not vaccines.
The snake oils are for no-brainers.
Don’t be daft : you don’t need any ‘medicine’ for a moderately severe virus like this one. We’ve never had ‘no interaction’ as a remedy for normal respiratory virus that’s not of high consequence.
Your alternatives of useless snake-oil or imprisonment are pure myth.
Especially when there’s no virus around!
One can use various well known ‘medicines’ for a variety of relatively mild virus infections, but they are cheap over-the-counter products; not good for the development of new ones!
“That medicine is either no interaction or vaccination.”
Utter nonsense. We know it’s nonsense because the “medicine” in all years before 2020 didn’t involve either lockdown or mass injection with experimental traetments.
You forgot the days when we were (and are now) below the average, think it’s called dry tinder. Various US states would like a word with your logic.
You cannot have “no interaction” in a modern economy. People would be starving within a week and dead within two.
Who do you think keeps the lights on and supermarket shelves stocked? The fairies?
11 million people have been going to work outside their home during lockdown and then returning to mix with the other 15 million people in their households.
Fairies united!
Yes and prior to 2020 – 90 deaths a day every day of the year from pneumonia – strange how seasonal flu and pneumonias have not figured in the figures if you get my drift/
Last April virtually all sick elderly inpatients were evicted from hospital and then virtually abandoned by the NHS. That is the major source of the inflated death figures you quote and of course many and probably most of the deaths attributed to Covid were no such thing. But of course, you know that already.
I’m sure Fon already knows that those figures confirmed as dead from corona are based on doctors’ paid-for opinion rather than evidence from autopsy.
Or: it’s already gone through the most vulnerable people in the population, and they’ve either died or survived.
Those under 60 may catch it and have mild symptoms. The number of people under 60 with no comorbidities who have died from CV19 is a tiny number compared to the whole population. The total number without comorbidities of all ages, according to the ONS FOI is <10,000
The deaths were high last year because it was a new virus that not everyone had immunity to. Care homes were badly affected after patients were discharged from hospitals into care homes without checking that they weren't infected.
The rules were changed on how CV19 were counted versus previous causes, meaning that ma were counted as CV19 which weren't, e.g. a GP putting CV19 on a death certificate for an elderly care home resident, without seeing the person beforehand, and because the person had had a raised temperature the week before. There are many, many causes for a raised temperature.
Worth noting the existence of the specific ‘glossary’ entry in the ONS weekly reports about how C19 can be classified as a cause. It’s the only disease that is segregated from the whole lot in those reports. We can look up either Covid 19, or ‘all causes’ on the ONS site. In simple terms, the accuracy of the certificates is open to question.
“This time last year we were having 1000 Covid deaths a day”
No, this time last year we were having 1000 deaths a day after people had tested positive using a test not fit for purpose. Even the WHO say that PCR should not be the sole method of diagnosis when a high number of cycles is used to magnify the viral fragment.
Until August last year, had Boris had an untimely encounter with a bus, he would have gone down as a Covid death. It wasn’t until those at the Centre for Evidence based medicine realised that no-one ever recovered from Covid in the U.K. (unlike in other countries on the Johns Hopkins spreadsheet) that they removed 5373 deaths from the U.K. total and brought in the “deaths within 28 days of a positive test”
Neither Michael Gove nor the CEO of the NHS could tell us how many people in hospitals were being treated solely because they had Covid and had been hospitalised as a result.
Even the CDC have recognised that the number of deaths with a positive Covid “test” in the USA has been grossly exaggerated since the method of recording deaths was changed.
Stick up your nose and
bodge the results and
throw your brain away:
nobody knows how they invent
their thousand deaths a day.
Thanks Annie, knew I could rely on you for a very apt rhyme!
‘Had Boris had an untimely encounter with a bus’ Stop getting our hopes up!
1. Cases and deaths collapsed last year over late spring and summer, with no vaccine: this is a seasonal virus as observed all around the world.
2. This time last year the eipdemic was at its height. This year it has run out of people to infect, due to herd immunity.
It’s over, fon.
We should remember that vitamin D deficiency is seasonal.
The body is designed to store up D in summer to be used in winter. If folk are not storing it, it’s because they’re not getting out there in the summer or slapping on too much protection factor whatever; thanks to MSM…. yet again!
Only for those people who do not supplement with it [in addition to getting good sun exposure during the summer months]
Its not the vaccines or the lockdown. Its season and herd immunity
A pictures worth a thousand words; well done!
Didn’t you say you were leaving yesterday?
1/ For the last 2 months or so, cases have declined across all age ranges – so vaccinated and unvaccinated cases have been falling.
2/ Deaths relate to infections which have occurred ~3 weeks earlier (incubation mean time ~5.1 days; symptoms to death mean time ~17.8 days).
3/ See 2
4/ See 2.
5/ Show evidence that outdoor hospitality is a diver of infection – thanks.
It could be vaccine. But you have no proof of this, you assert it like a minister but there is no evidence proving this.
In Texas cases have fallen there, and I doubt that cases are falling due to vaccines.
David Icke has suggested that the number of PCR cycles used in testing has been reduced causing the fall in cases.
It would be interesting to know what the truth is.
One thing I am sure of is that the vaccine deaths are far higher than reported in the US. In the UK the vaccine deaths are being covered up, we have no idea how bad it is. I understand that there is a facebook group of people affected by vaccine deaths. It will be interesting to see if they take it down.
I just learned of the sudden, shocking death from Mad Cow Disease of an energetic, wirey, 60 year old man. Lately, one’s vaccination status seems to have become everyone’s business, yet I suspect it will be considered very bad form to inquire about his.
Deaths Linked to Covid Vaccines are 30 Times More Common than with Other Vaccines – Is the
Safety Reporting SystemVaccine Fit for Purpose?It’s soft martial law, likely as preparation for the looming financial collapse that will lead to WW3.
Yeah, but the occasional clash with a vaxie fascist is rather fun. Or do I mean fon?
Both my parents are in their seventies and both have had the vaccine – the first one and the second one – the first one they had side effects for a day or two – the second one no side effects at all. I’ ve had vaccines before but I’ve decided not to have the covid jab (for the moment) – I’m under 65 and pretty fit and healthy (touch wood) and I really don’t feel as though I need to have it – I also suspect that I was probably one of those who contracted the virus very early on last year before it was even making headlines in the msm – l treated it as flu and even my local chemist told me that there was a flu-like virus going around – I got over it within a week then a couple of weeks later this flu-like virus called ‘coronavirus’ was hitting the headlines. I’ve decided to take Mike Yeadon’s advice and wait for a little while before deciding what to do. My gut feeling tells me that something is terribly wrong here – something not quite right – I can’t explain it but one motivating factor behind my decision is that I no longer trust this government to be honest – with so many twists and turns and so many decisions that simply do not make sense at all my trust in this government and in fact the entire political class is at an all time low and I don’t trust the lot of them to be honest ….but on the other hand I hope I’m completely wrong about this because so many family and friends have had the jab and I wouldn’t want anything to happen to them as a result of this damn vaccine. The are all completely understanding as to why I have refused the offer of a vaccination so far – but some have had no choice but to have it.
Ivermectin is a far safer treatment, and it can’t be less effective than the vaccine in terms of ARR. I know which I’d rather take.
Very similar to you, and I know some in their 70’s who are are happy with having accepted the offer. Here is what I said to the local surgery about my position at present. Re my comment in the third paragraph, there was no reply. They just put me ‘on the list’ of those declining the offer. They are not interested in finding out who is already immune.
I couldn’t agree with you more about A) Mike Yeadon – I read his podcast transcript he did with Delingpole and was chilled that someone with his background is smelling such an incredibly large rat – but I was glad in a way to hear him say it and confirm what we are discussing among ourselves and B) I shudder to think about the harms done to members of my family and friends who have completely unquestioningly submitted to this vaccine thinking that it would “protect” them or that it was the “right thing to do”. I also worry about the future “boosters” which all will be urged to accept with more sinister propaganda and the “cocktail” effect of such vaccinations, never mind the experimental gene therapy nature of what is being injected.
We’re not arguing amongst ourselves: neither Austin or fon are on our side
Video from Tim Spector ascribing fall in cases to vaccines.
https://www.youtube.com/watch?v=pD7V26exJuE
“video from Tim Spector confusing correlation with causation” – fixed it for you.
Yellow-card reports per 100,000 jabs of the AZ vaccine increased by 50% in early March, just when the mass media started reporting on blood clot problems with AZ. Clearly, during this time, there was no actual increase in side effects — this was a reporting system artefact.
The yellow-card reporting system is mainly a measure of trust in a given medicine/vaccine. It isn’t fit for purpose as a system of identifying side effects. This is particularly relevant for vaccines that have had their approval rushed through, where the full range of side effects might not be known, particularly for the more rare side effects.
I’d also note that the vast majority of scientific papers from the first half of last year that mentioned the potential for vaccines also suggested that there be a robust side effect reporting system, given the need for haste for approval. I don’t understand why this scientific consensus was ignored in favour of using the flawed yellow-card reporting system.
Toby, have you finally realised that these “vaccines” are dangerous?
I think everyone should stop ‘feeding’ these two ‘commentators’. Leave their comments unanswered, even unticked, they will soon tire of being ignored.
I postulate that the ‘vaccination process’ must be in trouble. The argument that there will be endless lockdowns if everyone doesn’t have the jabs is running everywhere. Its just part of a pysop and fear campaign that just might be running on empty.
As far as the vaccines are concerned, the effects are not reported efficiently or comprehensively, yet are still multiple times higher than other vaccines. And remember this thing has an IFR of 0.15%.
I have never had a flu jab, I am getting well on the way to 70yrs but have no intention of having an untested injection for something which the vast majority of healthy people of ALL ages don’t even know they have had.
“I postulate that the ‘vaccination process’ must be in trouble. ”
Well, let’s take a look.
The arrival here simultaneously of three pro-vaccine trolls rather underlines your hypothesis that the vaccination process is indeed in trouble.
Good.
“Even the previously vax-fanatic UK Prime Minister has signalled a change in the “narrative” by crediting lockdowns, not vaccines, for the recent collapse in cases and deaths.”
So, that being the case, if what he postulates is correct, surely it is the most massive counter argument to the proposed policy of introducing vaccine passports. If the vaccine doesn’t cause a collapse in cases and deaths what use is a passport saying you have had the vaccine?
“stop ‘feeding’ these two ‘commentators’”
Indeed – their ‘analysis’ is so daft, and clearly even the most basic principles of risk analysis and comparisons of treatment and control group data from a sample of a million are not understood.Nor the fact that these are experimental, only partially tested concoctions that required an expensive religious faith once they had been purchased sight unseen.
Looks like a poor wind-up.
They are complete 77th brigade distracting focus – I wouldn’t waste my energy on them never mind engage with them.
Deaths Linked to Covid Vaccines are 30 Times More Common than with Other Vaccines – Is the Safety Reporting System Fit for Purpose?
That is the sort of headline the liars and boneheads in the government nudge unit might concoct, to make you (like Boris has ) turn against the vaccines. Do not follow Boris.
Instead let’s look at the other side of the coin and check the truth about the benefits vaccine bestows rather than the extremely remote chance it might cause a problem. Once vaccinated the chance of catching covid19 and dying of it is 13 bn to one in your favour. here’s the breakdown, but no matter how you break it one risk in 30 versus 13 bn to one is tiny . Please do not believe the liars. Once vaccinated, your chance of contracting covid 19 and dying are 13bn to 1 in your favour. Hence please reject the dumb-arse anti-vaxx cretins.
https://www.youtube.com/watch?v=dr7AO2NvalI
The fresh data from America’s CDC indicates they’ve had 5,800 cases up to now where fully vaccinated people have caught covid19 (beyond 14 days after the last dose), these are called breakthrough infections. This is 1 in 13275, thus the fully vaccinated population would be around 77m, which I confirmed at ourworldindata.
Thus the vaccines they’ve used seem highly effective. Of the 5800 break throughs, 396 wound up in hospital (we have to assume they were mostly elderly since that is who has had the vaccine) and 74 of them died, which is ~ 1 in a million fully vaccinated people. Since 77m/74 =~ 1m.
So I think the IFR 1 in 400 (factor of 0.0025) versus 1 in a million is much better, esp. when one considers the fully vaccinated cohort is by and large quite elderly to whom the 1:400 ifr may be optimistic, 1 in 400 being the general ifr, including old and young people.
Note the 1 in a million is the chance a fully vaccinated person would die if they had a breakthrough infection. But the chance of a fully vaccinated person having breakthrough infection is 1 in 13275, which means: A fully vaccinated person of indeterminate age (probably old), but not presently suffering from covid19 has a 1 in 13,275,000,000, one in 13 billion chance of getting covif19 any dying from it (13.3k * 1m.)
Hence the vaccine is far safer than no vaccine. Do not be distracted by the knuckleheads.
cheers and good luck, fon
13bn to 1. Good luck with that
“let’s look at the other side of the coin and check the truth about the benefits vaccine bestows”
Have done. Base data from experimental surveillance with large controlled samples posted on this site.
Benefit (Decisive measure : absolute risk reduction) = vanishingly small = ~1%
Don’t forget to book your next ‘booster’, around July or August, because any claims you make for the vaxx will have run out by then.
The human immune system is safer and more long-lasting than a dangerous experimental medical gene therapy. The need for any COVID vaccine is obviated by the fact of available effective treatments for COVID. See compiled links to scientific information here: http://www.kathydopp.info/COVIDinfo/COVIDTreatments and http://www.kathydopp.info/COVIDinfo/Vaccines/NaturalImmunity
Great post kathydopp. Thanks for the links!
Lord Moynihan in the 1960’s summed up the difficulty of a generalised approach to infection describing that- “everyoperation is an experiment in bacteriology’” :and so it is with COVID19. Each individual has a risk with an infection or having a vaccination but unlike any previous vaccination program there data about either the infection or the vaccine is lacking . It is a matter of scientific disgrace that so many individuals have stood in front of the media and presented epidemiological models as inevitability and failed to recognise the risk of association and causation in data.
However for any young individual the use of a vaccine remains one which should never be mandated : at this time it is still an experiment.
The yellow card scheme is not fit for purpose and hasn’t been for years as my husband knows to his cost.
Part of the problem is this ” the system depends on the health care professional recognising that the symptom might be related to a particular drug, so if the connection is not made then neither is the report. ”
My husband and I had never heard of the yellow card scheme until 2020 and all the people who have been vaccinated had never heard of it.
Best video explaining why these COVID vaccines cause death via blood clotting
Doctor Bhakdi explains what happens after the human body is trained to produce COVID spike proteins in many more locations inside the body than the natural virus would have done. Young people who’ve been getting splitting headaches with the COVID vaccine are experiencing evidence of this serious blood clotting phenomenon. Every revaccination against a new mutant virus, will increase the danger for the vaccinated. https://www.youtube.com/watch?app=desktop&v=pyPjAfNNA-U&feature=youtu.be
Oh NO! Covid Vaccine is the Leading Cause of Coincidences!
A good friend of mine had the AZ vaccine and half hour later started having pins and needles in arms , hands, legs and then all over , then developed severe pain , shivering and numbness. She has been in and out of hospital for the last three weeks . The doctors were at first reluctant to admit it was caused by the vaccine , but the neurologist finally confirmed it , still no precise diagnosis but it seems it is a peripheral neuropathy and possibly vasculitis, an autoimmune response where inflammation of blood vessels attacks and destroys nerves .She is still in terrible pain and it is not subsiding. This was never listed as a possible side effect . The adverse reactions are not made known to people before taking the vaccine unless one does his/ her own research .