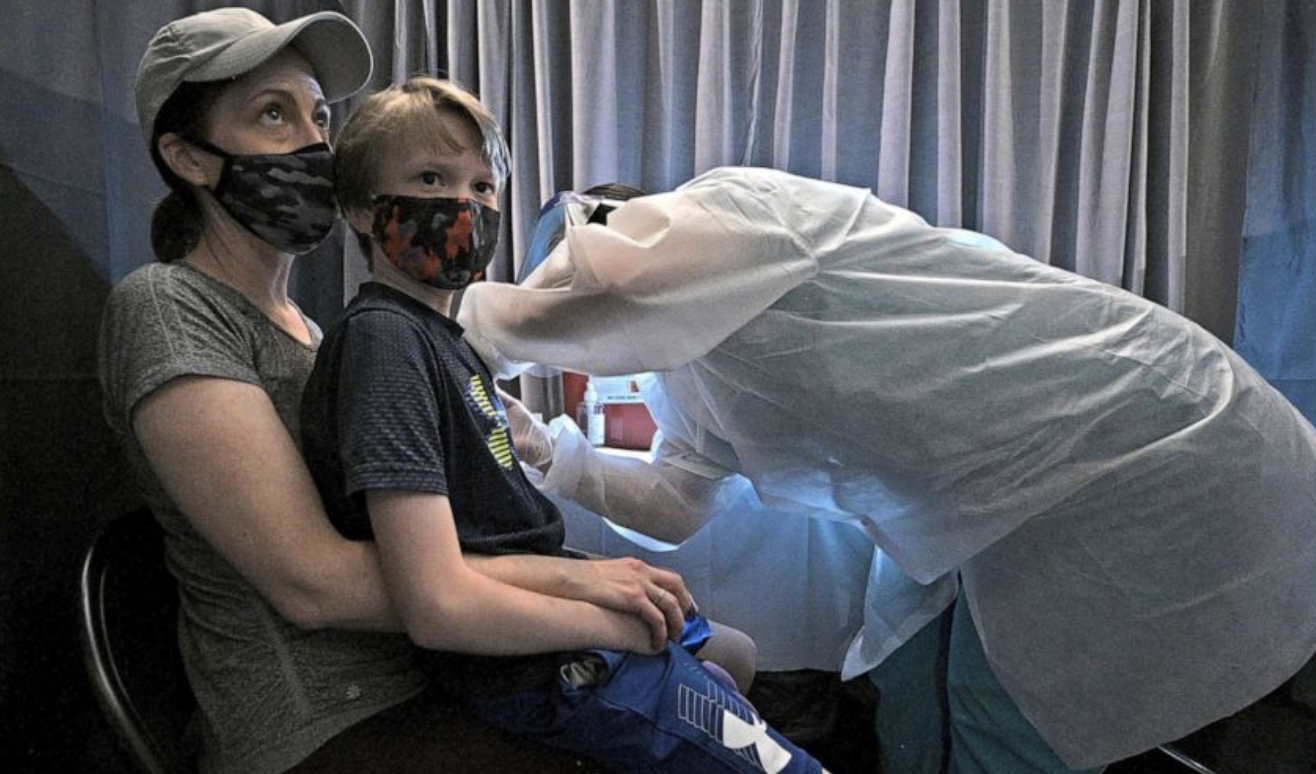
We’ve not yet finished debating the vaccination of 12-15 year-olds. But Pfizer’s CEO, who hinted at “annual re-vaccinations” against Covid over the weekend, is already looking to go one step further by asking the U.S. Food and Drug Administration (FDA) “in days” to authorise the use of its vaccine in even younger children. MailOnline has the story.
On Sunday, in an appearance on ABC’s This Week, Albert Bourla was asked when the country should expect the shots to be approved in kids between ages five and 11.
The New York-based firm, along with its German partner BioNTech, recently released data that it said showed the vaccine was safe and effective in a [sic] smaller doses in elementary schoolers.
“I think we are going to submit this data pretty soon,” Bourla told host George Stephanopoulos.
“It’s a question of days, not weeks, and then it is up to FDA to be able to review the data and come to their conclusions and approve it or not.”
According to clinicaltrials.gov, Pfizer’s study in younger children worked similarly to the way it did in older children and adults.
A total of 4,500 younger kids from ages six months to 11 years were enrolled at nearly 100 clinical trial sites in 26 U.S. states, Finland, Poland and Spain
About half of the ages five-to-11 group were given two doses 21 days apart and the other half were given placebo shots.
The team then tested the safety, tolerability and immune response generated by the vaccine by measuring antibody levels in the young subjects.
Pfizer said it had selected lower doses for Covid vaccine trials in children than are given to teenagers and adults. …
Bourla assured that Pfizer would be ready to ship these smaller doses across the country if the FDA authorises the shot in younger children. …
Unlike the larger clinical trial conducted in adults, the pediatric trial did not measure efficacy by comparing the number of Covid cases among the vaccine group to the number in the placebo group.
Instead, scientists looked at levels of neutralizing antibodies in young vaccine recipients and compared the levels to those seen in adults.
The companies expect data on how well the vaccine works in children between ages two and five and between six months and two years of age by the end of the year.
Worth reading in full.











To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
We must hide away our children until they can all get vaccinated. And afterward as well. We must hide them forever, because it isn’t safe. I know that sounds a little irrational, but it’s how I feel, and it’s also #science
Loon…..nay EVIL LOON
Do you have a temperature? Are you feeling a bit dozy? Because ‘a little irrational’ doesn’t begin to cover what you’re saying. I would worry about any children you were in charge of.
Six months old.
Six months.
Who the hell volunteers their baby for this??
People who have been indoctrinated by the State from the moment they entered nursery to the moment they left full-time “education”, I guess.
I follow Mumsnet a bit. They have a forum on Coronavirus in the health section. If you wonder who is volunteering their kids for this, just take a look at this forum. Typical post below, see a few of the responses.
Tell me why my son of 14 should have the covid jab? | Mumsnet
They have been completely brainwashed (although it is clear on the forum there are loads of trolls from pharma posting on there).
Some good posts on there including the one suggesting to watch this from 4hrs 13 – https://www.youtube.com/watch?v=bQevYc2jX7Y
When you go through the damning list of lies attached… is it any wonder that the British public’s mistrust and loss of respect for doctors is starting to edge out even it’s held in even lower esteem the corrupt politicians who’ve been assist these Davostokracy/GloboCap mandated racketeering operations?
What happened to the medical professions motto “first do no harm”? Those of them wedded to this BIG Pharma/Gatesian BUILD BACK BETTER narrative will increasingly be accomplices to mass murder. Why?
Because they criminally suppressed effective early treatments for SARS Cov2/COVID, and instead promoted the known iatrogenic fiascos of intubation and Remdesivir, this and they continue to mandate the nefarious PCR tests which have destroyed mass international jet travel…
Dial in failing to report experimental vaxx adverse affects via VAERS or the Yellow Card system – both through such defective websites that medics give up if they can be bothered to even report the harmful reactions at all – meanwhile the medical bureaucrats hide and game whatever data leaks through their mighty filters.
Not to mention the NIH funding – thanks to Dr. Peter Daszak – he who through EcoHealth Alliance developed the Covid-19 bioweapon in the first place and then swiftly helped launch toxic vaccines to neatly finish the job of despatching the weak, elderly and infirm off.
And now according to Dr. Leana Wen of George Washington University, she’s now stating that the vaxxd folk are a major threat to the unvaxxd because these jib-jabbed vaxxd are carrying higher viral loads, making them efficient Covid-19 super-spreaders. So there you have it my dear Skepticks… a “vaccine” that enhances disease transmission?
It’s almost as if they planned it don’t you think? And for sure you could of course claim Bozo and his merry band of Tory MPs get let off with their weasel-phrases to come of, “mistakes were made…” and “lessons learned…”
Though after making the first half-dozen mistakes or so, over the last 18 months you’d think these right-honourable politicos would simply just cop to their errors and now change course? You know make the shift back to life OLD NORMAL like it is in Norway, and has been in Sweden since the beginning of this plandemic.
That this isn’t happening is the biggest tell… Now we have petrol shortages, empty food shelves soon, and they’ll kill cash off when the can. Once the social credit score systems in its GAME OVER.
And yet there are still Mumsnet women out there who’ll be delighted to get their babies vaxxd ASAP?
Some of the posters on there on many forums make my blood boil! They ridicule and shame those who are pro choice or who declined the ‘jab’ and they are determined every man, woman and child should be injected. Gransnet is the same. They are both cesspits of fervent believers and trolls pushing the safe and effective narrative.
Some of them may pay a terrible price. Look at Maddie’s mother from the US Pfizer trial. She is living with her daughter’s pain now.
Bozo probably would! It would cut his expenses anyway.
The sort of people who followed ‘The Reverend’ Jim Jones to Jonestown and swallowed the Kool Aid when ordered.
Whether knowingly or through ignorance, these people are abusers of the children in their charge.
My daughter-in-law…
Why do we need to vaccinate children? They are not at risk from COVID and in any case we are not in a pandemic situation any more. That’s before we even get into the “adverse effects” arguments.
Is it because they’re running out of willing adults to take the experimental biological agent?
For Pfizer it’s just £££££s
For governments, it’s keeping the pandemic going, some combination of pure evil and utter insanity – hard to know the measure of each.
Well obviously this isn’t my opinion, but based on what I have heard from other Mums, it is the “Long Covid” myth that they have been convinced on now. Most accept that it will almost certainly be a mild and inconsequential illness, and that the jab won’t stop them passing it on. Some are afraid for their own health (mainly the fatties and the ones with the pseudo illnesses like fibromyalgia etc). But mostly they have been sold the lie that their kids will possibly end up with some kind of chronic illness. Prior to LC they were prob the sort who were convinced their kids had chronic Lyme disease.
So not only have the authorities played to hypochondriacs and OCD, they have made folks hydochondriacs for their children. The health service will so be overrun with these worried well! Is more and more of normal life being turned into a medical problem?
That’s it exactly. Tous les hommes bien portants sont des malades qui s’ignorent: all healthy people are sick, they just don’t know it. Recipe for total medical tyranny, i.e. total tyranny. Jules Romains predicted it a hundred years ago,
Long “vaccine” could be lifelong.
Quite!
So far around 130 000 people have died with covid (mostly not “from” covid).
So far over 1600 people have died from the “vaccines” and well over a million have been injured, some permanently. Similar figures from Europe and the US.
Going after children and soon babies. doesn’t bear thinking about.
Aaahh yes the hypochondriacs and ‘worried well’ who clog up the healthcare system
Nonsense graph – using a non-defined criterion of ‘excess’.
OK, show me a better one.
It’s a guaranteed catchment area, especially if their parents have already taken the plunger.
Its a disgusting dystopian project
Just be grateful they didn’t say each child has to have one eye removed to be safe from Covid.
If thy left eye give thee covid, pluck it out.
Shit, I was told it was the right eye. I’ll get the other one now!
You can be sure there are some who would do it.
“Instead, scientists looked at levels of neutralizing antibodies in young vaccine recipients and compared the levels to those seen in adults”
What a clever idea, because as well all know, small children are just miniature adults
Well that’s the only thing they can do as children are at zero risk of Covid
So any comparison with a control group would surely show no benefit
Its so outrageous, so criminal that I find it difficult to articulate my thoughts coherently. One thing is for sure I will never have any more vaxxines.
This will be the long term legacy of all this.
The irony of tyrannical behaviour is that in the end it provokes the opposite reaction it seeks.
It’s just too bad that we’re the generation that has to endure the tyranny and a future generation will reap the rewards.
That is exactly how I feel now too. I feel I would be very reluctant to have any medical treatment as what trust I had is gone!
Neither. When this is all over, there will need to be a truth and reconciliation programme to rebuild trust in medicine. Millions of people will never trust doctors and medicine ever again.
Exactly how I feel. I am no longer able to comprehend what I am seeing and can’t even describe it. There isn’t even any semblance of an argument for vaccinating children, meaning I no longer understand the actions and behaviours of my fellow humans.. a strange and awful place to find yourself.
I reckon that even those of us who are well aware of the history of the pharmaceutical industry have been gob-smacked at the ease with which they’ve been allowed to walk away with the swag.
Even the Pope woke up beside a horse’s head in his bed, as he’s now recommending the ‘vaccines’. Blackrock and Vanguard really know their stuff.
Spot on, as always, follow the money!
is this pope catholic?
“VATICAN CITY, Aug 18 – Pope Francis issued an appeal on Wednesday urging people to get inoculated against COVID-19, saying the vaccines could bring an end to the pandemic, but needed to be taken by everyone.”
https://www.reuters.com/world/europe/pope-francis-urges-everyone-get-covid-19-vaccines-good-all-2021-08-18/
What on earth does the Pope know about vaccines and immunology? It’s utterly ridiculous to say this. With a vaccine that doesn’t stop transmission it is not possible to stop the pandemic. Why has the Pope become a natural immunity denier? This all makes me so livid, it feels it won’t end. At least some people continue to say NO.
‘ … a vaccine that doesn’t stop transmission it is not possible to stop the pandemic.‘
What ‘pandemic’?
The Pope is a Jesuit and so he is a Globalist.
I assume it’s because he has bought the propaganda that it’s a safe way to save lives. He wouldn’t be the only one to be fair.
Certainly this doesn’t represent all Catholics, and is far from an ex cathedra pronouncement.
Do bears shit on toilets?
There is some anecdotal evidence https://www.msn.com/en-gb/news/world/alaska-woman-attacked-by-bear-up-through-her-toilet/ar-BB1dPZOO
Well l – I guess, by definition, he has a track record in making a career out of fairy stories and performative rituals of subservience. 🙂
So far as I can see, atheism is just another “religion”, with its own bunch of fairy stories. In any case, as my philosopher friend said, no such thing as certainty, just cumulative probability.
The answer to that is, no. Jorge Bergolio is not Catholic.
No, he’s a Jesuit.
Je-suis-It
Sadly not.
The so called pope (an anti-pope, heretic usurper) is and has been the evil plot’s biggest cheer leader.
Along with Dustbin Jellybaby, the worst archbishop any church ever had.
He certainly has a number of private opinions that many Catholics would disagree with. The same with Justin and the Anglican communion.
Apols..
Hat tip..The scaffold, Lily the Pink, 1968-
We’ll not drink a drink a drink-
to Billy the stinky stinky stink-
The wrecker of the human race..
He bought and paid for cytotoxic compound,..
..not efficacious in any way…
Does anyone still think that isn’t about depopulation?
How else are they going to get the US population down to 99 million?
This was first forecast in 2010:
https://www.youtube.com/watch?v=jY4Wahyo5n0
Whoever down voted this: keep living (for a short time) in denial. Eventually, the penny will drop.
So you seriously think there is a global conspiracy to drug people so they are infertile to bring the world population done?
And all pharmas are in on this? And all governments?
Well, obviously, there are a lot of greedy people who don’t know what the elite’s plans are and are going along with the tyranny for commercial gain.
But do you really think that the objective of jabbing EVERYONE on the planet is either for their health or to make even more money for big Pharma? For decades, members of the elite have let slip the depopulation agenda – it’s happening in front of your eyes and you still can’t bring yourself to accept it.
I think the key aim of mass vaccination is the passport – it is essential as it provides the gateway for blockchaining everyone. Given that the “project” is due for 2030 and beyond it makes sense for them to target the next generation. The young have always been their target but to keep with the narrative they had to provide it first to the elderly (after clearing a thousands of them out by pushing them into nursing homes knowing very well of the impact). Frequent jabbing will be necessary to maintain utmost control. BUt you’re right, any government or pharma that is willing to force this onto children whilst knowing the side effects vs reward is effectively carrying out an act of genocide in my eyes.
They are truly a religuos cult – somehow convinced that their doings are for a greater good and that a few dead/infertile/maimed children are nothing compared to the millions of deaths climate change will bring.
Not with me it won’t. The idea is nonsense. No power in the universe can curb human population growth.
A one-child policy dealt with it in China pretty decisively, compared to India.
Voluntary choices to have fewer children led to falling population in Germany, Italy and Japan. Good for them. Japan’s population peaked in the early 2010s.
Only Africa is now forecast to have any appreciable population growth.
I think you’ll find that injecting people with toxic substances will do just that.
Yes, we have a minor infestation of twitchy fingered jib-jab trolls on this site. I’m actually surprised it took them so long.
While trolls—to use a dehumanizing term—may be more likely to be manipulative, sadistic, and psychopathic, they may also be suffering, feeling lonely, and isolated with no clear socially acceptable outlets. Bless.
https://www.psychologytoday.com/us/blog/experimentations/201908/what-makes-internet-trolls-tick
There is also the paid version of course.
I’m kicking and screaming against this idea but running out of alternative explanations…
No need for anything outlandish. Money and power. Nothing new.
Very significant that they chose to measure antibodies rather than compare with a placebo test group. It surely couldn’t have anything to do with the fact that thre woudn’t have been any difference?
Indeed. The sustaining of the myths has largely depended on using indicators that aren’t.
I’ve been very sceptical about the depopulation theory until the past few weeks. But one really does start to wonder. The phrase that springs to mind is, “if something looks like a turd, smells like a turd…”
I simply cannot see any other rational reason for pushing experimental vaccines – which are known to cause all manner of adverse impacts – on babies and children to whom this virus represents zero threat.
There is no rational reason. The companies producing the vaccines want to make money. Hence, they want to expand the vaccination target group as much as possible. And the WHO no-trick-today-but-40-years-ago-you-should-have-seen-us!-pony wants to eradicate a disease with means unsuitable for this purpose, as always.
Why is it so difficult to understand that all these really stupid things being done all the time and resulting in havoc all the time are not conceived by evil geniuses with some hidden mystery agenda but by really stupid people being really stupid?
I think you’ve contradicted yourself. You set out perfectly rational reasons for the Pharma companies and the WHO to want to vaccinate everyone. Not stupidity, but self serving evil because they KNOW what they are doing is wrong.
What I meant to express is that there’s no medical reason to vaccinate 5 – 11 year olds, or no rational reason based on the assumption that medical professionals etc put the health of their patients first, something we usually supposed to take for granted.
No medical reason, indeed. It’s political and financial. The people pushing this are not stupid.
I’ll exclude the Pfizer guy who is just trying to cash-in on this opportunity as good as he can.
But the public health response to COVID-19, explicitly including the eradication policy the WHO is likely knee-jerkingly pursuing again, could be regarded as a definition of throwing the baby away while keeping the bathwater. And this is human stupidity at its finest. It’s yet unclear how much damage will still be done while trying to achieve an end which doesn’t really matter and is – very probably – inherently unachievable.
Nobody’s going to gain anything from that, not even the people who do push it: They’ll just end up in a state of failure with their respective reputations in tatters.
The story is, if people repeat lies often enough, they end up believing them. The dangerous thing is that people seem to believe that scientists are not flawed humans who can get things wrong like the rest of us.
Sorry, but I firmly believed this is a planned event. While I may not believe the depopulation theory (yet) for 2 strong reasons, there is no way this is just an uncoordinated mess.
Forcing people to pay for expensive PCR tests for international travel is not ‘stupid’ but planned to make money.
As I have said before, why are the free NHS PCR test results not valid for travel? Is this really about ‘a virus’ or is it about control and making money?
Face masks are a sign of obedience – all of the encouragement to wear them is not a ‘stupid’ act but a well thought out psychological operation to have the people prostrate themselves at your feet.
I have never been against vaccinations, neither have I been a ‘conspiracy nutter’ before, but I have felt since March 2020 that this whole ‘Covid affair’ is a scam, and I still do. The ‘vaccines’ and the closure of borders is only necessary to control people and to frighten them.
Face masks are a sign of obedience – all of the encouragement to wear them is not a ‘stupid’ act but a well thought out psychological operation to have the people prostrate themselves at your feet.
Face masks are a sign of European (here) people being no more intelligent than Chinese people in this regard: They do really believe this will protect them from dangerous sickness-radiation emanating from strange people. Probably more so, if everyone wears, them because dangerous sickness-radiation gets weakened every time it passes through a layer of cloth.
People have in no way grown more intelligent since the flagellants tried to appease God in order to rid themselves of the plague (and – of course – flagellants exist to these day, as I’ve just learnt).
Introducing them was a cultural copycat job. It’s been traditionally used in some places, such as the Tokyo underground – which is often crush loaded. Whether it does any good or not is a different question, but it looked like a good idea to some. “Something must be done” is, of course, the attitude of the charlatans in government.
“Something profitable must be done”.
Money and power are very rational reasons.
Bizarre why anyone would downvote this. Am I missing something?
Some people like red minus signs 🙂 .
All humans who do not pay their Danegeld to Pfizer are a threat.
The problem with the depopulation theory is that it is wildly speculative and may not come to pass.
And there is just no need to speculate about the future to call out this vaccine insanity for the monstrosity it already is.
The complete disregard for risk vs benefit is enough.
The insidious erosion of the body autonomy is enough.
The aggressive global roll out of a treatment that has no long term safety data is enough.
The coercion via vaxx passports is enough.
We already have enough facts and reality to work with without having to speculate about what may or may not happen in the future.
And anyone who claims to KNOW that this a depopulation exercise is just full of shit. It may be true. It may not. But nobody can really claim to KNOW.
The possibility of falling female fertility could be a cockup due to inadequate testing. This was caused in turn by the desperation to inject billions and make $$$$ before the virus faded away like SARS-CoV-1 did.
The >50 year trend from holistic medicine to ‘pharma-fascism’ seems to be a long-running trend. Medicalise all aspects of health, identify new diseases, scare people, all so that drug consumption rises. Read Malcolm Kendrick’s books Doctoring Data and A Statin Nation which set out how the statistics are faked.
That the compulsory regular medicine by “vaccination” programme necessary to engage in society could, in future, be used to require birth control injections is quite clear. State permission could be needed in future, perhaps even the near future, say 2030, to reproduce. A more effective programme than the one child tyranny, and one with which the vast majority – see Mumsnet – will go along with after blanket climate change fear porn propaganda. After a short time the sterilisation of the usual classes would follow, starting as usual with the disabled. Euthanasia of the sick and elderly would be presented as selfless, kind acts for which they would volunteer. (See Mumsnet.)
That would have been a ridiculous claim in 2019. Looking at events in Victoria, the speed of tyranny there – it’s absolutely possible.
I agree that it’s not necessary to believe that is the point to condemn what’s happening. It is currently counter productive to mention it is possible, let alone likely down the line.
SPARTICUS letter , if you haven’t already seen it.
https://www.zerohedge.com/covid-19/damn-you-hell-you-will-not-destroy-america-here-spartacus-covid-letter-thats-gone-viral
Crashes on me repeatedly.
This is a phenomenal piece! Thank you for linking to it.
Awesome article!! But the original PDF file has been deleted already! 🙁 😕 Do you know where else it can be found? I’ve copy-pasted the article itself for the record, but it doesn’t include the 14/27 pages of supporting references! 🙁
Try here. It has been archived. I have downloaded as a complete web page for viewing in my browser.
https://archive.is/CDc1J
Thank you!
Linked .pdf now gone. Archived here.
https://archive.is/CDc1J
This is absolute lies
https://medicalxpress.com/news/2021-09-covid-immune.html
It shows the MSM is proud to outright decieve.
We know that catching COVID provides VASTLY (18fold) better protection against re-infection than the clot shots.
Still the MSM hasn’t got around to saying “ecohealth alliance”….
This increasingly just looks like a cynical attempt to make money on the part of drugs companies.
They all have form when it comes to that.
What evidence does anyone have for thinking that they suddenly changed and have become moral guardians.
Its more than that. Its a way of bringing in vaccine passports and microchipping.
People are waking up to the fact that many of the politicians and the global establishments do not have your best interests at heart, many are utterly evil
I wish that were significantly true. I see no evidence.
Lots of unreported protests going on
My wife has a kid in her class who was flown to Germany by her parents to get the kab there a couple ofo the ago. They were desperate.
Some people are truly insane
Boris should put his 85 kids in the trials before anyome else should be required to have theirs jabbed.
I think it would b e safer for everyone if Pfizer had no involvement in the process at all.
Oh, and Pfizer — where’s the data from the original trials beyond the 6 month point? You’d think it would be important for everyone to know about how the original trial participants have been getting on, rather than hiding this information — at the very least, it makes people who find out that you’ve been withholding data suspicious that you might be hiding something.
I think anyone who takes any of these non-vaccines is an idiot and I have no sympathy for them at all. Injecting this stuff into children is simply sick and twisted. Are Pharma really that desperate for money? Perhaps not but greedy enough most certainly.
Gates and GAVI are behind all of this, I reckon. Pharma wants to sell vaccines, clearly, but Gates is the one who is keen on all the other bits of the globalist agenda.
“hinted at annual re-vaccinations”
I bet he did. Must be rubbing his hands with glee, even the government hasn’t been able to tax children – at best interpretation, at worst kill a few off. But why should children get in the way of profiteering?
They seem to have given up all attempt to persuade that this is the only protection against a deadly disease.
There are no words
So if children don’t really get symptomatic Covid, how do they tell whether the ‘vaccine’ stops them catching what they wouldn’t have caught in any case?
I guess they will just call it 97% effective (like they did the first time).
Were the 6 month olds given a full explanation which they could understand of the risks and benefits (not the big pharma version) or were they simply abused?
PURE EVIL…..child sacrifice…. 6uil 6ack 6etter
…lies damn lies, and statistics… sane shit, old story. Humans are delusional – it’s a Merry go round but I fear high centralisation and industrialisation = AI =The End
Infanticide.
Well who didn’t see this coming. They will introduce it for newborns soon.
What absolute mammary would do this to their child?
Of course they have it’s the obvious business model. Make sure everyone needs your product all their lives. It is a horrific prospect that the world is drifting into . When WHO and others said: no one is safe until everyone is safe-whatever the motive it made no scientific sense it was never a health objective but a business plan
If they only measured antibody response in the vaccine trials, then heaven help us!! How do you measure negative side-effects, i.e. its ‘safety’, via antibody measurement??
Folks should read this: https://www.docdroid.net/kZZXcGS/covid-19-the-spartacus-letter-pdf (via: https://www.zerohedge.com/covid-19/damn-you-hell-you-will-not-destroy-america-here-spartacus-covid-letter-thats-gone-viral).
I repeat…
WHY?
Does anyone know what the so-called benefits are? Is there a list available for scrutiny somewhere?
I’d love to see it.
The dangers are there, publicly available for all to see and they are feckin’ REAL.
How have we got to this? It’s completely unnatural. Humans don’t do that to their children, do they? Not in a civilized world.
These are our children/grandchildren FFS. What sort of parent would consider allowing such a thing without even a tiny bit of research?
Pure fuxxing EVIL on an unprecedented and epic scale.
Remember? 3 weeks to flatten the curve, freedom once the elderly and vulnerable have been stabbed, etc etc etc. Seems a long time ago, doesn’t it?
Back then if anyone had told you where the whole of the Western world would be by the end of September 2021 because of a respiratory virus whose overall survival rate is more than 99.8% of those infected, you would have accused them of being a full-blown tin hat-wearing conspiracy theorist. Wouldn’t you? I would have.
Blake Park Bandstand Bridgwater Somerset –
Sundays from 10 am
Meet friends – keep sane – we are awake.
Telegram Connecting Warriors
The unvaccinated are the much needed buffer to slow the mutating virus. By vaccinating anything that moves it is making things so much worse, but that is what happens when you let politicians and profits get in the way of public health and safety.