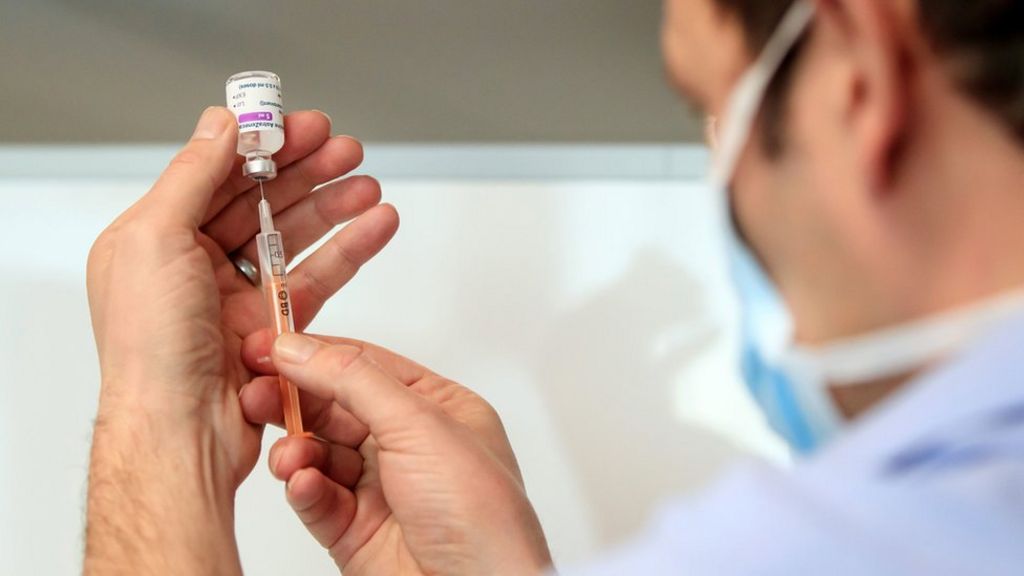
Ireland and the Netherlands have joined the list of countries that have suspended the use of AstraZeneca’s Covid vaccine because of fears over blood clotting. The Dutch Government has announced a suspension until at least March 29th. Both countries have paused their rollout efforts due to reports of “bleeding, blood clots and a low count of blood platelets” in health workers who had recently received the vaccine.
The Republic of Ireland’s Deputy Chief Medical Officer acknowledged in a statement that “it has not been concluded that there is any link between the Covid vaccine AstraZeneca and these cases [of clotting]”.
However, acting on the precautionary principal, and pending receipt of further information, the NIAC (National Immunisation Advisory Committee) has recommended the temporary deferral of the Covid vaccine AstraZeneca vaccination programme in Ireland.
The NIAC is due to meet again this morning. A further statement will follow thereafter.
AstraZeneca addressed these safety concerns in a statement issued at 6pm last night.
A careful review of all available safety data of more than 17 million people vaccinated in the European Union and UK with Covid Vaccine AstraZeneca has shown no evidence of an increased risk of pulmonary embolism, deep vein thrombosis or thrombocytopenia, in any defined age group, gender, batch or in any particular country.
So far across the EU and UK, there have been 15 events of DVT and 22 events of pulmonary embolism reported among those given the vaccine, based on the number of cases the Company has received as of 8 March. This is much lower than would be expected to occur naturally in a general population of this size and is similar across other licensed COVID-19 vaccines. …
Furthermore, in clinical trials, even though the number of thrombotic events was small, these were lower in the vaccinated group. There has also been no evidence of increased bleeding in over 60,000 participants enrolled.
One particular batch of AstraZeneca vaccines (which is involved in reports of a death) was sent to 17 countries, an increasing number of which are putting the rollout of this vaccine on hold.
Stop Press: Health officials in Northern Ireland have announced that they will continue to use the Oxford-AstraZeneca vaccine after its suspension in the Republic of Ireland.













To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
“Because it was his own staff association that was taking action against him, they would not fund any legal advice or action in support of his defence…“
…Straight out of the Franz Kafka playbook diligently followed by yet another captured institution – Add the Police Federation to the long list of unions and professional bodies adept at representing themselves, but less inclined to represent their members.
The irony is that I’d bet good money they wouldn’t have sacked him if he’d not been white. I know that had he been ‘BAME’ he’d hardly have been likely to have made these comments in the first place but it’s now abundantly clear that as long as the racism is geared towards white people ( by coloured people or other whites, shamefully ) then it’s all good, because nowadays it’s repackaged as ”positive discrimination” most of the time, and that’s acceptable, according to Clown World logic. But whistleblowers/dissidents must be quashed and silenced at all costs. A tale as old as time.
It would be remiss of me not to conclude that the little khant’s brown fingers are all over this. And the envelopes too.
I think the Khant would ethnically cleanse London of white people if he could.
That’s in train isn’t it?
Absolutely.
Sir Knife Crime is doing a pretty good job of it anyway.
Banned from standing is the crucial point here. They don’t dare let the members decide whether what he did or said merits dismissal. I’m sure whoever made this decision claims to believe in “democracy”.
The members should leave the federation.
I think we all know what ”anti-racist” is code for nowadays, don’t we?
”£60k for an “Anti-Racist Practice Lead” at Norfolk County Council.
As a Norfolk MP, I am calling on @NorfolkCC
to dump this poisonous role.
End the ideological rot and focus on delivering efficient services for my constituents.
We don’t want, or need, this political garbage.”
https://x.com/RupertLowe10/status/1915700901353840667
If only he could lead Reform but then that is Nigel Farage Limited which has just taken cash from a former Tory funder who is a Nigerian born Lebanese muslim. Talk about shitting on your support base.
All roads lead to Marxism in today’s woketastic Clown World, and there appears to be little available in the way of an antidote;
”Europe has genuflected to Islam while America has bent the knee to multiculturalism; both are arguably the same. The engine is Marxism, and we’re seeing that what can’t be won at the ballot box is doing amazingly well in the streets and growing in power and acceptance in our courts.
Marxism is more than a textbook definition; it is an always morphing state of mind with only one defining quality: the subservience of the individual to the State and/or an authoritarian that knows best. Whether people call themselves progressive, leftist, anarchist, socialist, or communist, among other names, all are ideologies that seek to control people through whatever narrative serves them best.
Today, that narrative is multiculturalism, a cultural reworking of economic Marxism. The goal is to restrict individuals’ ability to think and act in their best interest in favor of the imperial “they,” with few knowing who actually pulls the strings (visualize Biden’s Trojan Horse presidency).
The connective tissue of multiculturalism uniformly weaves a misleading tale of caring for the downtrodden and those who, for whatever reason, aren’t enjoying being part of a country of free and self-reliant people. Part of that connective tissue is incipient hatred for the successful, especially independent thinkers and entrepreneurs. We see this vividly play out in violent and illogical demonstrations on our campuses and streets by uneducated young people led by professional agitators.
The beauty of the left’s hatred is that it never has to be based on provable truths; it just must be believed, much like any other religion that demands faith over provable fact. While not a religion per se, it takes on many of the same trappings, fooling weak and uneducated minds with popular narratives in place of actual knowledge and truth.”
https://www.americanthinker.com/blog/2025/04/multiculturalism_s_marxist_roots.html
So, there you have it folks…
Not even trying to hide it any longer
So it reduces to the Police Federation Boss being sacked after having the courage to tell the truth that is, in any case, clear for all to see. British society, employers, institutions, as well as Policing, are rife with precisely the dynamic that he so well describes.
If you live in a British city and you aren’t concerned about self-defence ten you are ignoring reality. The use of knives has increased dramatically and will increase a lot more in the near future. Different mindset when you have to assume that everyone is tooled up.
Through a present red wine haze I seem to remember in the run up to my retirement from the Met a whispering campaign to set up an alternative to the Met Federation.
There are two main reasons to join the Federation; legal representation should the sh1t hit the fan, and secondly for some of the financial benefits that come with membership (discounts at certain retailers, investment plans, and such like).
Were I still in the Job, I would absolutely consider that alternative because I wouldn’t want to send another penny to the ar5ehole5 at the official Federation.
Who actually sacked him?
Did all of the members hold a ballot?
As I understand it, the “rank and file”members like and support Mr Prior and they had no say in the decision to sack him, are they wrong?
Perhaps the 30,000 members could grow a backbone and withdraw their membership and subs?
The Federation should represent its members so the Federation should support whoever is elected by the members, and not ban someone from running for election.
Unless they are not interested in representing their members and are pushing an ideology.
Every institution is captured. This is the danger of consolidating great power into the hands of a few. For the weak, offices of governance is a draw to power they would be incapable of achieving on their own merits alone. The solution is for freedom loving people to contest appointment to these offices and drive the scumbags out.