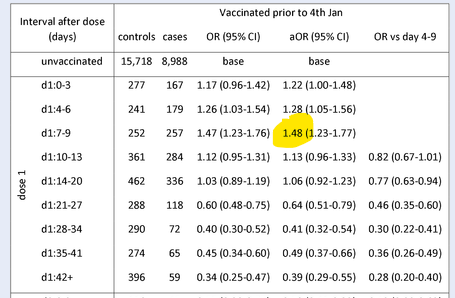
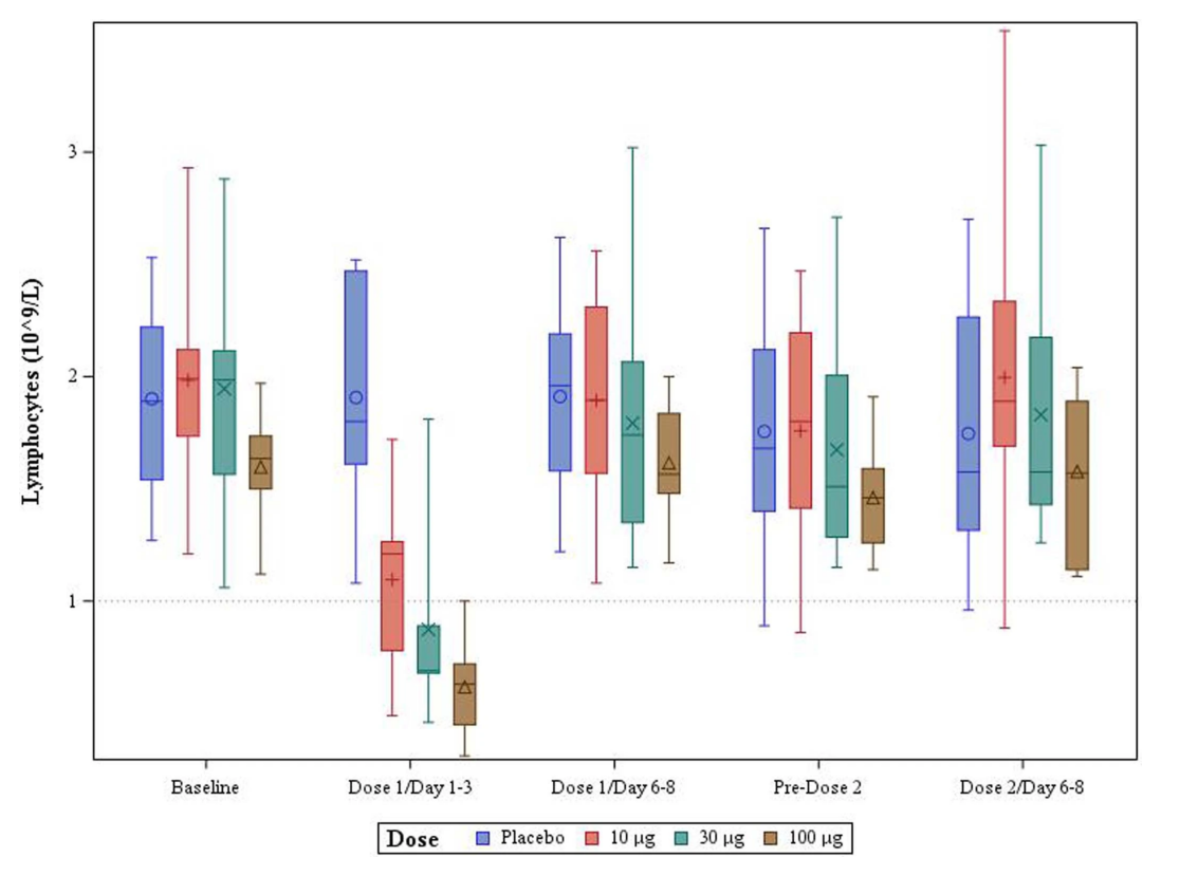
Lockdown Sceptics reported last week on two studies that had found an increase in COVID-19 infection risk in the week after the first vaccine dose. A PHE study found a 48% increase in infection risk in the over-80s group 4-9 days after receiving the first dose of the Pfizer vaccine (see table below). And the American FDA Emergency Use Authorisation for the Pfizer vaccine found 40% higher “suspected COVID” in the first week after vaccination compared to the control group. As a potential explanation, Lockdown Sceptics noted that in trials the Pfizer vaccine was found to suppress lymphocyte count in the first few days after treatment (see chart above), potentially increasing susceptibility.
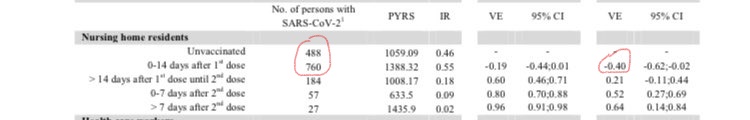
Now a new paper from Denmark has made a similar finding. Tracking every vaccination given to nursing home residents, it finds a 40% increase in infection risk in the 14 days following the first Pfizer dose (see table below). (Ultimately, it finds a 64% vaccine efficacy for nursing home residents a week after the second dose.)
Does this explain why there have been numerous reports of care home outbreaks shortly following vaccination? Could this be why Dr Hervé Seligmann found an elevated death rate among vaccinated people in Israel?
Is anyone in Government or the MHRA asking these questions, if only to rule out any problems?
Personally, I would be reassured to see the overall mortality rate of vaccinated and unvaccinated people over time. I cannot see why it is not routine to collect and publish this data, though it appears it is not. This would be the most straightforward way of establishing whether there is a potential problem that needs addressing in relation to short-term vaccine safety or there is not.
Will the Government publish this data to set our minds at ease?










To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
“The people have failed us, Comrades. We shall elect a new people.”
Adam Grant makes a very interesting point, although I can never see it happening in practice. Democracy is more than elections. Electoral systems are producing poor/mediocre leaders throughout the world (remember Hitler got to power through popular vote) and in the USA something close to civil war. Sortition is a democratic alternative. There is no reason why the pool of candidates should be approved to match WEF ideals. As Grant says the test of competence might be something like a civics test — the same standard as immigrants applying for citizenship.
Who defines what a “poor/mediocre” leader is?
I don’t know much about “sortition” but you’ve got a problem with defining the pool of candidates – who decides on the candidates? – and you have a problem with the fact that those willing to do the job will be of the same type as those who currently apply. The issue as I see it is mechanisms for limiting state power, and checks and balances, not hoping for some mythical perfect or “good” leader.
I’m happy with letting the people decide. It’s up to us to win the battle of ideas, though the playing field is not currently level because private corporations have become political and/or aligned themselves with the state.
I think the idea that what we need is better leaders is a big source of our problems. Hoping for a saviour might be emotionally gratifying but it gets us nowhere. I actually think it hurts us badly.
100% agree. Of course some are more honest and competent than others, and when that’s obviously the case we should choose them in preference to others, but most of them are not like that at all and never will be.
I have to repeat this tof:
Our salvation will not arrive via the ballot box.
Exactly, HP. Unfortunately we will have to suffer this system until it collapses completely under its own hubris. Some say don’t vote but that doesn’t really work as the numbers not voting will always be minimal and it allows some complete shyster into parliament.
When politics became a career rather than a vocation, it went downhill at a rate of knots. Witness the House as it is now – all there as they are incapable of productive work in the real world
In the UK we used to have a multi layered system of selection in which largely independent constiyuency associations of parties could chose pretty much who they liked as canddiates. they would promote and support them and the people would chose who to elect from the options provided by parties or independents.
It was as a result of such local independence that Churchill survived his challenge to the defeatist Conservative government in the 1930s. In tose days local Conservative associatioins had hundreds or thousands of members and even the Liberals had dozens or hundreds. Labour had fewer and relied on support from unions and the Coop movement.
Nowadays the central party selects potential candidates according to the values of the leadership. That is why we have so many Cameron clones on the Tory benches: people who have never worked, never taken a personal risk and don’t know what either capitalism, markets or women are. Constituencies are often in the position of selecting the least worst from their point of view.
Good point. That was probably a better system. The constituency associations should never have accepted the imposition of candidates – they should just have quit/formed a new party, en masse.
Anyone seeking power needs to be independently assessed for their fitness to govern or represent the people. This should include psychoanalytic testing to root out any potential rotten apples. I know that sociopaths can do a good job of pretending to show empathy/compassion so maybe before anyone becomes eligible they should undertake public work. I like to imagine people like Blair and Johnson having to dig trenches, being garbagemen or doing care work for a year. Extreme I know but somehow we have to rid elections of self-serving barstewards who think they can swan out of Oxbridge and, after a few years, straight into government.
A working democracy requires the citizenry to participate. That, as De Tocqueville noted, is why it worked so well for so long (pace slavery and post-slavery Jim Crow) in America.
That participation is collapsing all over the Western world. That and a functioning democracy means the majority buying into a common culture. And “culture” is by definition MONO – as it belongs to a specific group of people – “multi-culti” in reality means no culture, the fracture of the existing culture – and a nation that loses its common culture has had it.
As we have. FUBAR. I might say, at 72, that it won’t worry me for long, but both sides of my family are very very long lived*. Guess I’ll be out there on the barricades at some point, the way things are going.
Of my three GPs, all Victorian born, one died at 70, WWI wounds – the others, 102, 96 & 96
remember Hitler got to power through popular vote
He didn’t. The Weimar end game began when the last parliamentary coalition government (Kabinett Müller II) broke down in 1930. This was followed by a string of governments ruling by decree based on the power of the president of the Reich to issue emergency decrees parliament could reject but didn’t need to approve.
The first two were led by Herrman Brüning (Zentrum) who managed to get a negative coalition together: While the parties of the Reichstag couldn’t agree on forming a coalition, the majority agreed on tolerating Brüning’s minority government by not voting against his decrees. Brüning ultimatively stumbled over the Great Depression and reparation payments dicated by the treaty of Versailles. After him, Franz v. Papen was appointed chancellor but Reichstag wouldn’t tolerate his government. The subsequent cabinet led by Kurt v. Schleicher suffered the same fate. By that time, the NSDAP had become the largest parliamentary faction but it didn’t have enough seats for form a government of its own. Hitler was appointed chancellor by the president on strong recommendation of v. Papen and originally led a coaltion government with mostly conservative (ie, monarchist) ministers.
Yes but the NSDAP got to be the largest party because more people voted for it than any other party (and traditionally the chancellor was the leader of the largest party).
Hitler didn’t get to power because of the popular vote. He was appointed as leader of another minority government backed by the power of the Reichspräsident to issue emergency decrees. The NSDAP never won an election in the sense that it would have been able to form a parliamentary government of its own and its vote share actually decreased in the last ‘free’ election of the Weimar republic. The enablement act got passed because the parties in Hitler’s minority govenment (from memory, DNVP and NSDAP) secured support of the Zentrum (Catholic party, recreated as CDU after 1945, presumably at least partially as a cover-up¹)
¹ That’s my private opinion on this. The official reason is that the new party was meant to be open to Catholics and Protestants alike.
Elections are a threat to democracy.
Just one big, big problem with that statement – we do not live in a democracy.
And for confirmation that we longer have democracy a read of Alex Klaushofer’s article on 15 minute cities will provide all the proof required – nobody voted for the bloody things.
You’re absolutely right.
What they’re really worried about is an outbreak of actual democracy.
Thanks
Too true, HP. We live in a quasi-dictatorship. Nothing that we say is important is ever taken seriously and they bit by bit are eroding our freedoms. They are certainly not making our lives easier.
While not disagreeing with you, HP, I think the problem is a bit more subtle / nuanced.
Yes, we definitely do not have direct democracy (like Switzerland) where people get to vote on specific issues – we did not vote for 15 minute cities.
But direct democracies are rare. The more usual form is representative democracy where voters elect representatives to take legislative decisions on their behalf. And, though parties in this system do publish manifestoes ahead of elections, they have never been seen as comprehensive or binding, certainly not legally so. It could be argued that 15 minute cities are consistent with representative democracy.
Representative democracy breaks down when representatives no longer act in good faith and rather implement measures they know would never be voted for in a direct poll – such as 15 minute cities, mass immigration, net zero, ULEZ, etc.
Allied to this, we have an effective uniparty system, with both main parties following similar policies across a broad range of issues, and where those policies are not supported by the voters. And first-past-the-post ensures that the uniparty cannot easily be challenged by newly-established parties (such as the Dutch farmers).
“Representative democracy breaks down when representatives no longer act in good faith and rather implement measures they know would never be voted for in a direct poll – such as 15 minute cities, mass immigration, net zero, ULEZ, etc.”
Thanks for your response Michael but this comment rather confirms my point of view.
Thanks, HP. We are not disagreeing…
https://www.conservativewoman.co.uk/trafficked-raped-and-humiliated-the-poor-white-girls-of-rochdale-cast-aside-by-police/
This makes grim reading. While the focus of the article is on Rochdale I can state categorically that if we ever get a proper investigation in to Child Sexual Exploitation (CSE) in Oldham a similar horror story will be revealed.
Currently we have Bunter Burnham blocking an investigation and the reason – the rotten and useless GMP otherwise known as Burnham’s enforcers.
Yet further examples of “democracy.”
The situation in Telford is not exactly encouraging either. Furthermore, anyone who draws attention to Telford is likely to be harassed by the police.
Harassment by the police is also Burnham’s preferred method. A friend of mine severely embarrassed Burnham at a public meeting, the next day a squad car arrived at her house with three plods who basically threatened her. She ended up leaving the area and moved to Yorkshire.
Do you think there is a “back-door” from senior local authority employees to the police? I recently attended an “in camera” inquest, held with press excluded ostensibly because of respect for the feelings of the deceased’s family. When certain issues were raised, the LA tried to cover up some dirty washing which I knew of and thought might be relevant. I tried to raise the subject with the coroner at the time, but he would not allow evidence to be given which might challenge the evolving “official narrative” about the deaths and the negligence of the LA therein. A few days later the police visited me without prior warning and threatened me with arrest over an entirely different matter involving a paranoid neighbour who is easily triggered into making false allegations against me and others in the neighbourhood and was well known to the police. It was reasonably clear to me that the police had approached the neighbour who was was all too quick to make an allegation, albeit minor, against me which the police “felt” obliged to investigate. Just conceivably, the timing of their visit was a co-incidence, as the neighbour had been behaving like this for years; just conceivably, the neighbour chose that moment to make the allegation; just conceivably the police accessed early reports from the neighbour, but why? I made a formal complaint, and we arrived at a “fudge” and I let the matter drop. “Just a coincidence: no evidence.” Since that time I have occasionally wondered if the coroner or the senior LA employees had had quiet word with the police, but, more importantly, what dirty washing were they covering up? The UK establishment never ceases to amaze me in its ability to use one part of it to cover up errors made by other bits, especially between senior staff. However, the trend is now to harass MPs, public sector employees and even private citizens who blow the whistle, or merely fail to enthusiastically support the establishment agenda. (There should be a Bates cartoon titled “The MP who failed to applaud the Party Chairman at the annual conference.) ” They now find themselves quietly “disappeared” on social media, their employment is threatened, and a smear campaign is mounted against them.
Do you think there is a “back-door” from senior local authority employees to the police?
No question about this.
Only a few years ago OMBC gave a job to a senior copper within GMP – the boobgate woman – she was clearly ruff as well as useless. GMP “let her go” ie she was sacked. OMBC took her on at a salary of £120k, more than the police salary and her job – Director Police Liaison. We never had a Director Police Liaison. It was a non job and they have since had to “let her go.”
I would be amazed if Burnham wasn’t involved in this.
Local Authorities are unbelievably corrupt. I would be here all night if I started detailing the stories. Most people haven’t got a clue.
https://www.conservativewoman.co.uk/revolving-doors-at-climate-change-central/
And here is an example of more “democracy” in action courtesy of the “fill yer boots” grifters populating the Climate Change Committee at great cost to the rest of us and I don’t mean just their salaries.
With droughts, storms and other extreme weather getting worse around the world,’ she disinformed, ‘people who have done the least to cause climate breakdown need nations with high historic emissions like the UK, to accelerate the energy transition.
This is such a bunch of lying stewards that they wouldn’t ever be tolerated in a bar. Historic CO₂ emissions don’t matter. Whatever damage they might have done, this has already happened and we’re irrevocably living with the consequences. What matters is current and future emissions because these are the ones supposed to cause climate catastrophe. Hence, it would absolutely imperative that countries currently causing lots of CO₂ emissions must reduce (China, USA, India being the top-most but the UK is far distant from the 4th position) these quickly if this was really about avoiding an imminent catastrophe. Transitioning anything in the UK to anything else is not going to make one jot of a difference. Time and money wasted on that is time and money wasted insteads fighting the imminent catastrophe.
Considering this, these people obviously don’t believe in their own rethoric, no more than the partying Downing Street crowd believed in a deadly pandemic. They want money. Lots of it. And they want to get it from rich ‘soft targets’ like the UK where even the most inane propaganda, say, about a deadly pandemic which isn’t really causing any deaths, is going to be trusted because all the official authorities have been hijacked by all the vested interests.
https://www.conservativewoman.co.uk/stop-forcing-these-deadly-dangerous-evs-on-us/
And here is some more democracy in action – trying to force dangerous EV’s on the population. These four wheeled bombs are going to kill people.
Never mind, “lessons will be learned.“
After the uprising of the 17th June (1953)
The Secretary of the Writers Union
Had leaflets distributed in the Stalinallee
Stating that the people
Had forfeited the confidence of the government
And could win it back only
By redoubled efforts. Would it not be easier
In that case for the government
To dissolve the people
And elect another?
Bertolt Brecht
Die Lösung / The Solution
There are all sorts of things to consider in terms of demographics. The difference between the boomers and the younger people is very stark and not at all healthy and probably the most important consideration in the Anglosphere. Some of the bommers are moving into their dotage which brings to mind Gramsci when he talked about the dying of the old and yet the new cannot be born and so morbid symptoms appear.I can say with certainty that the near future, say the next ten years will be very different given the mindset of the young.
Let’s face it. We are led (sic) by an Incompetent bunch of politicians. Pretty much all ” navel gazers” with average IQ’s
“average IQ’s”
Surely a gross over statement.