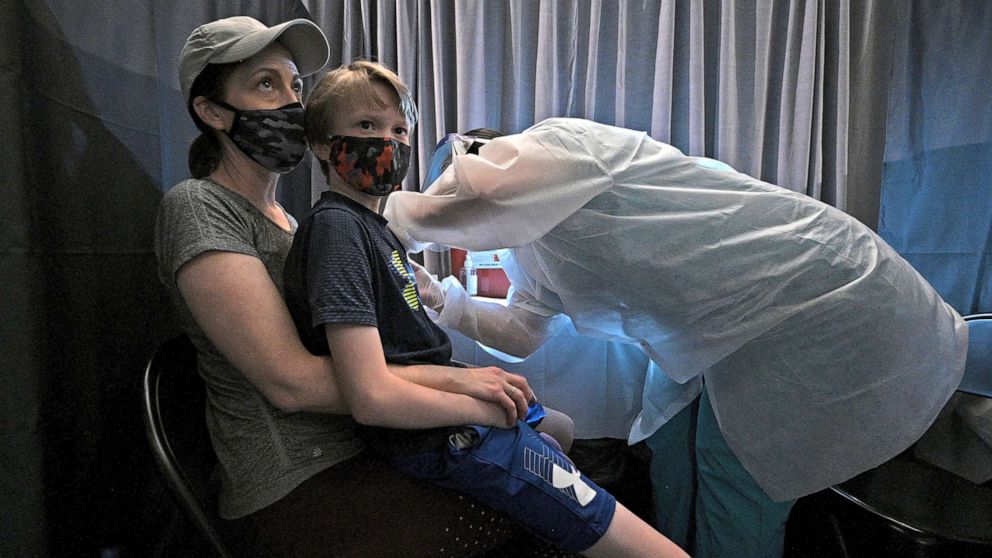
Professor Nigel Curtis, from the Murdoch Children’s Association, has warned that those under the age of 11 must not be given a Covid vaccine until more data becomes available. Professor Curtis also remarked that, as the virus poses little to no risk to young children, the safety threshold for the vaccine has to be much higher than it is for older age groups. The Mail Australia has more.
On Tuesday the U.S. approved the Pfizer vaccine for children aged five to 11 years-old after a study of 3,100 kids detected no serious side effects and found it was 90.7% effective in preventing Covid infection.
Pfizer has started an application process to get its vaccine authorised for children in Australia, but Professor Nigel Curtis from the Murdoch Children’s Association believes bigger trials are needed first.
Professor Curtis said children are much less likely to suffer severe disease than adults so the safety threshold for the vaccines should be set much higher.
“I’m a strong proponent of vaccine in general but when we decide on the use of any vaccine we always have to weigh up potential benefits and potential risks”, he told ABC radio host Virginia Trioli on Friday morning.
“That balance is much more difficult than it is in older age groups and the main reason is that in this age group, even with the Delta variant, Covid remains a very mild or even asymptomatic disease in the vast majority of children”.
Australian data from the first seven months of 2021 showed 2.5% of children under nine who caught Covid needed hospital treatment, compared with 7.7% of adults in their 20s and 19.2% of adults in their 50s.
Professor Curtis said vaccinating under 12s was “still an open scientific question” and “we need more data on the relative benefits and potential harms”.
“The bar for using a vaccine for a disease which is extremely mild and which very few children come to any harm is much higher”, he said.
The children’s doctor said U.S. trials had involved “a very small number of children when it comes to assessing rare side effects” and “you need much larger numbers before you can be certain”.
Phase three of Pfizer’s vaccine trial for adults in November 2020 enrolled 43,661 participants, while AstraZeneca’s safety data was based on 20,000 adults enrolled across four clinical trials.
“Children are not little adults and they have a very different immune system. They have a very strong immune system when it comes to Covid and they respond very differently to adults”, Professor Curtis said.
Worth reading in full.











To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
It really is a shame we can’t turn back the hands of time. Then we could be more like Hungary. They called it and are much better off as a result;
”Hungarian Prime Minister Viktor Orbán’s hard-line immigration policy has been vindicated amid the terror attacks and demonstrations witnessed across Western Europe in the aftermath of the Hamas attack on Israel earlier this month, a leading U.S. conservative journalist has claimed.
In an interview with The Critic, U.S. editor and author Rod Dreher told the magazine’s executive editor Sebastian Milbank that Orbán had been proven right in his decision to oppose mass migration from the Arab and Muslim world, citing the pro-Palestine protests and the rise in anti-Semitism experienced across many European nations.
“One thing I can say about Budapest is that, whatever else is happening in Europe with radicals in the street, Islamists on the street causing mayhem, it doesn’t happen in Budapest,” Dreher said.
He explained that in the aftermath of the Hamas terror attack on Israeli territory on Oct. 7 in which 1,300 civilians were slaughtered, “an intelligence analyst friend back in Washington texted me and said that we were about to see the vindication of Viktor Orbán’s migration policy, and he was right.”
Dreher, who recently moved to Budapest after falling in love with the Hungarian way of life, noted that “tens of thousands of people across Europe turned out to support Hamas.”
Although qualifying this observation by stating he does not equate supporting the Palestinian cause with supporting Hamas,” he added that “it seems to me that in the days immediately following the butchery in Israel, to go on the street and demonstrate generally in favor of the Palestinians, with no criticism at all of Hamas, I think this is a distinction without a difference.”
https://rmx.news/hungary/orban-was-right-hungarys-immigration-policy-vindicated-in-aftermath-of-hamas-terror-attack-us-conservative-rod-dreher-claims/
If only I spoke Hungarian, I’d consider a move. To be in a country that doesn’t have immigration problems sounds peaceful. I’m sure the dark powers that be are already plotting to bring down the Hungarian government though.
Many speak English there now, I had no problem. It’s taught at schools, so younger people speak it, and many older. You’d be fine. Go! You can always come back.
Perhaps I’m missing something, but if you move to Hungary is that not….immigration for them?
Yes, Spuddy, you are missing something. Aethelred would no doubt be happy to adopt Hungarian ways as would I. There is such a thing as good immigration, neutral immigration and bad immigration.
Yes, it looks like the dark powers have now got their man in place in Poland.
I think it’s fine to want to limit immigration and preserve the culture. I’m all for it.
But is the population ready to accept the economic consequences? I don’t think it is.
What do mean by ‘economic consequences’, Stewart?
Well, there would be many, short term and long term.
In the short term, all else remaining equal, there would be a drop in population as total immigration exceeds population growth and has for many years now.
There would be in particular a drop in the working age population.
And that would mean that wages would go up, so goods and services in Britain would become more expensive.
Britain has a gargantuan debt, like most western countries. That debt would become harder to service with a shrinking working population.
Don’t get me wrong. I’m all for it. If it were up to me we would slash the size of the state, starting with all the benefit schemes that discourage work and get more of the people already here working.
But I don’t think the population is up for that. They want the fantasy of all the goodies from the state and no immigrants and I don’t that will work.
The vast majority of immigrants are not net contributors to the public finances.
And that puts the tin hat on pro immigration arguments.
Source?
I’m not aware of any UK studies by ethnic group – they probably wouldn’t get any funding – but studies in both The Netherlands and Norway found that only immigrants from other equally economically-developed states made net contributions to current government expenditure. Afghans, Somalis and Roma were the worst performers.
I can’t think of any reasons why it would be different for the UK, but perhaps you have the stats?
When we take into account the fact that immigration is increasing the population, which means extra immigrant taxes have to cover both extra current expenditure and the extra capital expenditure required to add to infrastructure,it makes it even harder for them to be economic assets. Especially as they have more children and are more likely to have non-working wives.
We’ve had 26 years of by far the highest immigration in our history. Taxes are at their highest for a lifetime, public debt is at record levels and growing rapidly, public services are deteriorating and our infrastructure is collapsing.
Look we can do all the mental gymnastics we like. You can cherry pick ethnic groups that are not net contributors. You can conflate whatever economic data you like (Japan also has the highest debt ever in its history with virtually no immigration).
The reason that the state lets in immigrants in droves is because it is desperate for economic growth and it knows that one very simple tool to achieve it is population growth, which with a low birth rate can only be accomplished with immigration.
People can. get as angry as they like at the notion that less immigration will have negative economic consequences, but it just will.
To be honest, this is a bit like with Brexit, which was riddled with fantasy and wishful thinking. People were sold lie after lie after lie, immigration this, NHS that, trade deals galore.
All bullshit. `Annual immigration has more than doubled since the referendum. The NHS is an even bigger shambles than it was. There are no trade deals.
I’m not saying the EU isn’t a monstrosity and the project wasn’t worth a try. But anyone who wanted to be honest with themselves knew perfectly well that none of the things that were promised were ever going to come to pass, amongst other things because our political class is worse than useless.
Tired of immigration, of the ethnic, cultural, and religious transformation of Britain. Fine. I don’t disagree. Just don’t pretend it’s not going to cost something to stop it, when it’s so blatantly obvious it will.
I can’t speak for anything else..but I have family and friends in Lincolnshire and they definitely rely on immigrants to do farm work..picking food, canning, planting etc……they have done for 20-30 years…
I think up to 50% of the workforce is immigrants..and that rises to 90%+ during harvest….but these habe historically been from Eu and Europe….
…and you are right, a lot of people don’t understand how much certain industries rely on them.…
it’s the way the UK works…….because the UK has a fairly lightly regulated labour market with a large low-wage sector…we have a consumption led growth model which depends heavily on household consumption and population growth, rather than exports…for instance about half of Germany’s GDP comes from exports …our is less than 30%…
…this strengthens demand here for migrant workers who help to consume those goods and services…
While countries like Germany have a strong export-oriented manufacturing base, the United Kingdom relies more heavily on services, not only high-skilled (e.g finance) but also low-skilled sectors (retail, cafés, restaurants, personal and social services) and the construction sector. These sectors depend to a larger extent on migrant workers, especially in low-paid employment…like pickers and packers, where about 40% of them are foreign…
So it isn’t a simple as just stopping immigration..although we all agree I think that the unregulated flood going on right now, is not the way to manage anything….it also clearly matters where they come from, and do they want to work..?
“To be honest, this is a bit like with Brexit, which was riddled with fantasy and wishful thinking.”
Ignorance at its finest.
Brexit, if done per the wishes of the electorate would have been the reset this country needed. We were denied Brexit because the people running the country, and that does not refer to those treasonous pantomime dames in Westminster, had decided that a resurgent Britain, property led, would be a threat to their “Great Reset.” The United States of Europe was going to be so much easier to manage and then those awkward, bastard Brits put a spoke in the works and to make matters worse The Donald took the USA. The Great Reset was decidedly looking a non-starter and the Davos Deviants were in a right effing pickle. What shall we do? Fortunately, for whatever reasons China agreed to help out with some crappy actors collapsing on film and whoosh the C1984 was up and running. And here we are.
“Immigration drives economic growth.”
A new modelling scenario brought to you by Professor Pantsdown of Imperial College, London with funding from the world’s greatest philanthropists the Bill and Melinda Gates Foundation.
Brexit was a nice idea. But it had no chance.of success because it was a fantasy.
The public have spent the entire post war era voting for fantasies that keep being promised to them.
Like long retirements with generous pensions. Or plentiful, high quality medical care for all. Or benefit after benefit for those who can’t be bothered to work.
Voting for big state administered projects that sound wonderful is not smart. Its aive really.
Why would you believe that the class of people who have driven the country into trillions in debt could successfully deliver on all their Brexit promises?
And what level of naivety does one have to have to think that an establishment that is against the project and thinks it has about half the country on its side wouldn’t do all it can to sabotage it all?
Being realistic about what can and cannot be achieved is not ignorance. Quite the opposite.
Read my post:
“We were denied Brexit because the people running the country, and that does not refer to those treasonous pantomime dames in Westminster, had decided that a resurgent Britain, property led, would be a threat to their “Great Reset.”
stewart, Forgive my reprinting two of your posts, but for me it’s the easiest way to respond. (You in grey, me in red).
(approximately a day ago):
“Well, there would be many, short term and long term.
In the short term, all else remaining equal, there would be a drop in population as total immigration exceeds population growth and has for many years now.
There would be in particular a drop in the working age population.
And that would mean that wages would go up, so goods and services in Britain would become more expensive.
Britain has a gargantuan debt, like most western countries. That debt would become harder to service with a shrinking working population.
Don’t get me wrong. I’m all for it. If it were up to me we would slash the size of the state, starting with all the benefit schemes that discourage work and get more of the people already here working. But I don’t think the population is up for that. They want the fantasy of all the goodies from the state and no immigrants and I don’t that will work.”
I don’t agree. I think that many people are up for a smaller state, exactly because it will mean an end to all those goodies and precisely because it might get people back to work. It depends on who “they” is, in any case. I don’t receive anything from the state – but they sure take a hell of a lot from me – and give it to people who in many cases one way or another just piss it up the wall.
(in response to someone else’s post):
“Look we can do all the mental gymnastics we like. You can cherry pick ethnic groups that are not net contributors. You can conflate whatever economic data you like (Japan also has the highest debt ever in its history with virtually no immigration).
The reason that the state lets in immigrants in droves is because it is desperate for economic growth and it knows that one very simple tool to achieve it is population growth, which with a low birth rate can only be accomplished with immigration.”
I don’t have a problem with your economic growth argument – it sounds very reasonable to me (although there are surely ways of getting our own feckless population up off the couch, before using foreign a workforce). But do you really believe that far-seeing politicians concerned about economic growth is the only reason so many illegal immigrants are entering the country (at such risk to themselves as they clamber into small boats)? Really? Politicians thinking long term – a touch disingenuous? Perhaps, if that’s true, politicians should engage the public and argue the point intelligently? Your arguments may well be correct – I’m no economist – so, given that the politicians don’t engage the public and argue the point, the rather inescapable conclusions are either that they couldn’t give too hoots about economic growth, or that they have another agenda – or two agendas. Unimaginable! I mean, again, we’re talking about illegal immigration, right? So make them legal! Don’t force hundreds of them into small vessels, to drown at sea.
But politicians couldn’t give a toss; they’re in the grip of something bigger – and this is no doubt where the real disagreement between us lies – something external that yearns for borders disappear and for what we’ve understood Britain to be to disappear too. What is this thing? Some say the WEF; some say the Long March – which some would say are the same thing, sort of. The extent to which you disagree with that notion will depend on your age, perhaps – and whether or not you remember this country as it was, warts and all – at a time when, with all its undoubted faults and problems, it didn’t feel it needed to be part of some larger political entity in order to solve its problems.
“People can. get as angry as they like at the notion that less immigration will have negative economic consequences, but it just will.
To be honest, this is a bit like with Brexit [groan], which was riddled with fantasy and wishful thinking.”
And so to your comments about Brexit, which I’m afraid I’ve encountered many times before.
I think perhaps that you underestimate the people who sign in to this website; most are/were not “riddled with fantasy and wishful thinking” when entering the voting booth. Some ‘lower-calibre’ Brexit voters no doubt were of that kind, but I don’t think most were. I voted to leave because I was certain that whatever swivel-eyed crooks we were forced to vote for under our present system would be less swivel-eyed and less croooked (and even removable should we want to remove them!), and at least more democratically-minded than the continental brand, and that the tremendous campaign of deception undertaken 50-odd years ago by Heath et al was always bound to meet its nemesis – because that’s what usually happens to relationships formed through lies. When I voted to leave I was clear in the understanding that we would probably suffer economically. I even thought it might be a severe blow; but I didn’t think for a moment that there wouldn’t be ways around the problems that might arise. Things weren’t appalling until a certain obscenely propaganised virus came along, then suddenly we were taught the real meaning of economic destruction. Now we’ll never know what Brexit would have either achieved or inflicted. I wish I had a pound for every time a Remainer has told me I’m a racist, riddled with fantasy.
“People were sold lie after lie after lie, immigration this, NHS that, trade deals galore.
All bullshit. `Annual immigration has more than doubled since the referendum. The NHS is an even bigger shambles than it was. There are no trade deals.”
Bullshit for sure, and I’ve no time for Brexiteers who thought that all sorts of cures and solutions would inevitably flow from leaving the EU. But I’m sorry, we’ll never really know, will we. I wonder just how much of this shambles has been caused or exacerbated by that thing…what was it called now?…. oh yes – lockdown. Besides, the facts that “annual immigration has more than doubled since the referendum”, and that the “NHS is an even bigger shambles than it was” are not criticisms of the principle of leaving an entity as gruesome as the EU; they’re criticisms of the morally bereft nature of our politicians and of the unprecedented and profoundly damaging turn of events that we’ve suffered over the last three years. How is it possible to ignore this minor development?
“I’m not saying the EU isn’t a monstrosity and the project wasn’t worth a try. But anyone who wanted to be honest with themselves knew perfectly well that none of the things that were promised were ever going to come to pass, amongst other things because our political class is worse than useless.” See above. Some Brexiteers were idiots – most weren’t. Most were realists tinged with a philosophical sense of resignation.
Tired of immigration, of the ethnic, cultural, and religious transformation of Britain. Fine. I don’t disagree. Just don’t pretend it’s not going to cost something to stop it, when it’s so blatantly obvious it will. Again, I don’t know who you’re talking to there, but it’s not me or any of the people I know. I’m not sure I ever met any of the ones you’re thinking of.
Brexit was a nice idea. But it had no chance.of success because it was a fantasy.
Again, this is only partly true. It was a fantasy in the minds of some, but if you voted in the hope that the EU might be kept at bay, and that, even at the cost of the opposite of the fantasies you refer to, some independence as a nation might be retained, then Brexit has in fact been a success. You see, we’re still not part of the EU, despite many efforts to change that situation. (And taking a look across the Channel, I’d like to suggest that that isn’t exactly a bad thing…)
The public have spent the entire post war era voting for fantasies that keep being promised to them. Like long retirements with generous pensions. Or plentiful, high quality medical care for all. Or benefit after benefit for those who can’t be bothered to work. Voting for big state administered projects that sound wonderful is not smart. Its aive really. Why would you believe that the class of people who have driven the country into trillions in debt could successfully deliver on all their Brexit promises? And what level of naivety does one have to have to think that an establishment that is against the project and thinks it has about half the country on its side wouldn’t do all it can to sabotage it all?
Tend to agree with you there, but the Remain camp’s post-vote shenanigans took even seasoned campaigners by surprise. It took many Remainers by surprise too. It wasn’t “naivety” that made people think they could trust a system that had never let them down so outrageously before; it was a centuries-old, subconscious belief that they lived in the world’s most civilised country, where it was taken for granted that such things simply didn’t happen. With what happened at that time, and with what happened subsequently during the Covid farce, they’ve wised up. They’ve rumbled the politicians, who will never regain the questionable respect that they once enjoyed. Now, everyone watches their back.
I remember talking to many Remainers and to many Leavers before and after the referendum, and after we actually left, some four bloody years later. What struck me most was that most Leavers, fantasising or not and with the exception of a relatively small number of morons, cared about the principles involved: self-determinism, nationhood, democracy… while Remainers cared principally about their material prosperity. How you voted determined which trumped which. It’s as simple as that.
Ignore comment following this one!
I don’t have a problem with your economic growth argument – but do you really believe that far-seeing politicians concerned about economic growth is the only reason so many illegal immigrants are entering the country? Politicians thinking long term? Really? Perhaps politicians should engage the public and argue the point intelligently! Your arguments may well be correct – I’m no economist – so, given that the politicians don’t engage the public, the conclusions are either that they couldn’t give too hoots about economic growth, or that they have another agenda – or two agendas! I mean, again, we’re talking about illegal immigration, right? So make them legal! Don’t force hundreds of them into small vessels, to drown at sea. But politicians couldn’t give a toss; they’re in the grip of something bigger – and this is perhaps where the real disagreement lies – something external that yearns for disappearance of borders, and of Britain itself. Some say it’s the WEF; some refer to the Long March – not unrelated, I suppose. The extent to which you disagree with that notion will depend on one’s age, perhaps – and whether or not one remembers this country as it was, warts and all, at a time when it didn’t feel it needed to be part of some larger political entity in order to solve its problems.
Indeed your comments about Brexit are not entirely unfamiliar… I think perhaps you underestimate many people – such as those who sign in to this website; most are/were not “riddled with fantasy and wishful thinking” when voting. Some idiot Brexit voters no doubt were of that kind, but not most. I voted Leave because I felt that whatever swivel-eyed crooks we had to vote for under our present system would be less swivel-eyed and less crooked, and more democratically-minded than the continental brand, and that the campaign of deception dreamt up years ago by Heath et al was always bound to meet its nemesis – because that’s what happens to relationships built on lies. When I voted, I understood that we would probably suffer economically. I thought maybe be severely so; but I didn’t ever think that there wouldn’t be ways around problems that might arise. Things weren’t in a particularly bad way until Covid came along, when suddenly we were taught the real meaning of economic destruction. Now we’ll never know what Brexit would have either achieved or inflicted. I wish I had a pound for every time a Remainer has told me I’m a racist, riddled with fantasy.
Then you say that “Annual immigration has more than doubled since the referendum. The NHS is an even bigger shambles than it was. There are no trade deals.” Yes, bullshit for sure, and I was as baffled as the average Remainer by Leavers who thought that all sorts of cures and solutions would inevitably flow from leaving the EU. But again, we’ll never know. Besides, the facts that “annual immigration has more than doubled since the referendum”, and that the “NHS is an even bigger shambles than it was” are not criticisms of the principle of leaving the EU; they’re criticisms of the morally bereft nature of our politicians and of the unprecedented and profoundly damaging turn of events that we’ve suffered over the last three years. How is it possible to ignore this minor development?
Brexit was a “fantasy”? Only partly true. It was a fantasy in the minds of some, but if you voted in the hope that the EU might be kept at bay, and that, even at the cost of the opposite of the fantasies you refer to, some independence as a nation might be retained, then Brexit has in fact been a success. The thing is, we’re still not part of the EU. (And taking a look across the Channel, I’d like to suggest that that isn’t exactly a bad thing…)
Tend to agree with you about “naivety” – but the Remain camp’s post-vote shenanigans took even seasoned campaigners by surprise. They took many Remainers by surprise too. It wasn’t “naivety” that made people think they could trust a system that had never let them down before; it was a centuries-old, deeply-held belief that they lived, as their forbears had lived, in the world’s most politically civilised country, where such things simply didn’t happen. With what happened at that time, and with what happened subsequently during the Covid farce, they’ve wised up. They’ve rumbled the politicians, who will never regain the questionable respect that they once enjoyed. Now, everyone watches their back.
I remember talking to many Remainers and Leavers before and after the referendum, and after we finally left. What struck me was that most Leavers – fantasists or not and with the exception of a small number of morons – cared about the principles involved; self-determinism, nationhood, democracy… while Remainers cared principally about their material prosperity. How you voted determined which trumped which.
See my post above. From Migration Watch
Try again
That’s very very funny, regardless, don’t give up the day job. These figures will only have got worse, by a large degree.
https://www.migrationwatchuk.org/briefing-paper/347
“Response to UCL paper on the fiscal effects of immigration to the UK30 December, 2014
Summary
Overall cost of migration
1. Between 1995 and 2011 the fiscal cost of migrants in the UK was at least £115 billion and possibly as much as £160 billion according to a report from the Centre for Research and Analysis of Migration headed by Professor Christian Dustmann at University College, London. The report found that migrants in the UK were a fiscal cost in every year examined.[1]
Contribution of ‘recent migrants’
2. The report claims that migrants who had arrived in the UK since 2000 had made positive contributions throughout the period from 2001 to 2011. This does not appear to be correct, the figures in the paper show that the contribution from these recent migrants was negative in each year after 2008.
Contribution of ‘recent A10 migrants’
3. The authors also highlighted a finding that between 2001 and 2011 recent migrants from Eastern Europe had made a net contribution of £5bn. While this correctly reports their most optimistic finding, their calculations in four alternative scenarios were all lower. One of these alone was enough to reduce the contribution to as little as £0.066bn – a sum within the margin of error of such calculations.”
Ok you got me.
An organisation whose stated mission is to reduce immigration to the UK has produced a paper that shows that migration to the UK produces a fiscal deficit.
Yep, that pretty much ends the debate right there.
We’ll ignore all of history and the track record showing increase population leading to increase economic activity (which is practically a tautology). We’ll ignore the historical records showing how population declines through famine or war led to subsequent periods of poverty. We’ll also ignore Japan, the only modern day case of a country that has declined in population due to low births and no immigration and seen a relative economic decline during that period, as everyone expected and predicted. We’ll just pretend there is no link whatsoever between the population of a country and it’s GDP, none at all.
And we’ll just go with your paper from the completely objective, not at all biased in any way Migration Watch UK.
Over the last two decades Japan’s population has declined by 2%, while the UK’s has grown by 14%. The difference between the per capita GDP growth rates of the two countries over the same period has been negligible, around 0.1% per year. You’re assuming some kind of major downturn which isn’t there.
But there is no problem with a drop in population. Infact net immigration is running at about 600,000 a year. These people will all have to live somewhere and presumably that will be in a HOUSE. But we are about 300,000 house short already. Isn’t it funny how Japan isn’t flooded with migrants and still functions? Isn’t it funny that Poland and Hungary are not flooded with migrants and still function but we CAN’T. ——Or WON’T. ——We are an ill disciplined rabble governed by political dreamers fantasising about being “world leaders” in everything that does us no good whatsoever, like pretending to save the planet. ——How about if we become “world leaders” in border control? ——Now isn’t that a novel idea?
“world leaders” in border control?”
Now that would be a valuable export.
I’m not talking about still functioning. I’m talking about prospering and thriving.
Japan has a declining population and declining wealth. Which doesn’t mean it is a bad place to be. But it is poorer than it was.
They’ve clearly made that choice and perhaps are happy with it.
All I’ve said is that there would be economic consequences. Its hilarious to see how many people seem to find just that simple statement of fact objectionable.
I agree with you. People obviously read something else into your comment or we have some trolls voting en masse.
Japan has a declining population and declining wealth. Which doesn’t mean it is a bad place to be. But it is poorer than it was.
USA
South Korea
Poland had a slightly declining population over this period, in which its per capita GDP more than doubled
and South Korea did that with a population growth less than half the UK’s. Perhaps we can learn something from them there, after all.
China
…and China had around half the UK’s population growth over that period. It also experienced population decline in the last 3 years of that period, but kept growing strongly. China also has a far smaller proportion of immigrants than Japan or the UK
Agreed. When politicians say ‘poorer’, what exactly do they mean?
I always think these sorts of arguments are misleading because they don’t actually reference the quality of life of ordinary people, which is more to do with social connection, quality of housing, purpose, connection with nature etc. Who exactly is ‘poorer’ and in what way?
Perhaps if you are so easily upset by people seemingly disagreeing with you maybe you should not discuss controversial issues. But in actual fact I don’t disagree with you. I am adding to the conversation and I have pointed out that I feel the western worlds politicians are pandering to the one world government people at the UN and WEF who see open borders as a goal because when you destroy national identities and culture you end up with populations that feel more like citizens of the world (They call it the International Community) rather than citizens of individual countries. people in France no longer feeling French, Germans no longer feeling German etc etc and all just citizens of the world all governed by unelected technocrats. ——Ok so there is a case for immigrants that are required for certain types of work, but the situation is out of control and if it was all really about economic growth we would not be getting rid of cheap abundant energy and replacing it with expensive unreliable energy, since availability and price of energy is what drives growth as we see from the German example where they plastered their country in turbines and have the highest electricity prices in Europe along with Denmark and ourselves who followed the same anti capitalist eco socialist path. ——PS try not to go in the huff mate. People disagree with me often as well you know.
Eh? Easily upset? What are you talking about?
I’m just arguing my point that cutting back on immigration is likely to have some negative economic consequences and that I don’t think that those who advocate for much less immigration realise it.
And to be honest, the negative reaction has done little to persuade me otherwise because all I’ve seen is a bunch of irrelevant comments that amount to “we’re fed up of immigrants, they’re wrecking our country and our culture, enough is enough.”
The only relevant argument I’ve seen is jeremyP99 with a paper from Migration Watch UK. I don’t think a paper from a lobby group constitutes a strong argument, but at least it’s relevant.
What the high cost of wind turbines have to do with the economic impact of a potentially shrinking population is not immediately clear to me. Neither is how the French or Germans feel about their nationality. Nor the UNs push to create one world government.
You do realise that one can be against mass immigration and still accept that without it the population and the economy could shrink (or grow much more slowly), right?
No, you are not just arguing your point. You said “it is amazing how people find a simple statement of fact objectionable”. ——-You are indicating you are displeased that someone else has a different idea. I don’t find your constant focus on economic growth and immigration as the only issue. There is also the issue of culture, national identity etc to consider when importing masses of people at these rates when we cannot find enough houses for the people who already live here, and also the issue of the overcrowding of a country who has now overtaken Holland as the most densely populated country in Europe.——-I mentioned wind turbines which really includes all of the green agenda in relation to your remarks about economic growth and migrants, because renewable energy has a negative effect on economic growth by causing huge price rises and it is well known in economics that availability and price of energy is directly connected to well being , prosperity, health, life expectancy and ECONOMIC GROWTH. Germany who have about 40,000 wind turbines have greatly increased their electricity costs and that has damaged their industry and economy. We in the UK are on the same absurd path. So if it is economic growth we should be concerned about, and I agree we should be, then why import millions of migrants supposedly to improve economic growth and at the same time force up energy prices to lower it.
I always laboured under the assumption that GDP growth has absolutely nothing to do with productivity growth. Isn’t it productivity per capita that enriches a nation. On the international tables we haven’t done well for two decades or more. Politicians are happy to bamboozle people with the myth that if GDP is expanding we are better off and thus immigration is needed.
Also we know now that multi-culti is total bollocks. For a start, culture -m which apples to a specific group of people, is hence, by definition, resolutely MONO.
Multi-culti = NO culture. We have imported millions, many with no desire to integrate (Quran FORBIDS integration with us kuffir), with their families (same), and benefits a gogo on tap. No contribution to access benefits.
Which is why we now have thousands crossing the channel in small boats. The streets of ENGLAND are paved with gold, from their point of view, funded by the ultimate sucker, us the taxpayer.
Yes, I know that having lots of immigrants from places with very different customs changes the country. And in ways that I don’t find very appealing either.
Do you think that that somehow changes the economics of immigration? It doesn’t.
I don’t have to delude myself that there are no economic consequences to agree that I don’t want a particular type of immigration. In fact, I’d rather know.
Stewart you cannot have it both ways. ——You say you don’t find this mass immigration of alien culture very appealing but then you say we need it for economic growth. ——-Perhaps if we didn’t hand out welfare like confetti and got our own people off the couch to pick the berries and potatoes we would maybe cut the need for a few million migrants. ——-But with this endless growth you talk about we should all be concerned that the whole country ends up like London in 50 years time with no room to swing a cat.
What most people do not understand is that the total of working age people on benefits in this country is 7 million and rising.
That’s a totally different matter.
If you want to get rid of benefits for people of working age (with only very small extreme exceptions) I’m right behind you.
I can absolutely not find mass immigration unappealing and recognise its impact on economic growth.
That isn’t having it both ways. Having it both ways is having a shrinking population and having sustained economic growth. At least relative to others.
Maybe it’s possible, but I don’t think so and there is very little empirical evidence to support it.
Stewart —-I think we have covered this issue now. We agree on some things and not on others. That is the good thing about this site.
Absolutely agree. And don’t forget that economic benefits from, ahem, let’s say those excitable chaps who want us to integrate with them and live under Sharia, are only measurable by GDP which measures “benefit” from prostitution, drug dealing as well as rather more genuinely beneficial activity.
Compare and contrast with Polish plumbers who either want their kids to be (old fashioned) British, or who come here avowedly temporarily, saving up to buy a nice house when they return to Poland (as a very large number already have.)
Any discussion of economic benefits or disbenefits which doesn’t recognise the difference between the two groups belongs to those lovers of unicorns and kindly fairies.
And also note that those who can only acknowledge the alleged “disbenefits” of a slightly warmer world since the end of the Little Ice Age, such as the “Unprecedented” weather events (actually “unusual” at worst) and who advocate spending Trillions on non-solutions to non existant “problems” are not to be believed about anything at all.
Yes good points. ——-But I will leave my harping on about the climate change eco socialist scam for another article, as I already post extensively on that issue and don’t want to bore the pants of everyone when this article is supposed to be about immigration.
Selective Skilled immigration enriches a nation via productivity growth. We are simply growing GDP via increased money supply, consumption and services. GDP growth is positive if it is matched by increased productivity per capita but it ain’t in the UK and hasn’t for quite a while.
It needed saying Jeremy.
You suggest that a growing population is in some way associated with economic well being. I have never heard of such a theory. Please will you provide the URL links.
As to a falling population, I do not think that is a problem for a homogenous society. I do not think it is a problem at all. We are told several conflicting things by the politicians who just want to create anxiety:
A
the population will fall so fast we cannot man the critical services. The rate of aging does not support this and, besides, they have taken so much of our money over the decades sionce the NHS came in we must all be fitter longer to continue working. Indeed, we will have to continue working because the ponzi scheme for pensions does not work.
B
AI will make us all unemployed.
C
All manufacturing not already in China will go there so there will be no jobs
D
Therefore, the politicians say, we need a lot of immigration.
Of course population growth leads to economic growth.
You van argue that you prefer less population growth at the expense of economic growth. But to argue that population growth doesn’t lead to economic growth is just silly.
It’s not the ONLY thing that produces economic growth, but it’s one of the things that does.
That people should dislike the simple and pretty uncontroversial fact that the bigger the population the bigger your economy pretty much proves my point that many. people just don’t want to face up to the consequences of less immigration.
They think they can have their cake and eat it.
I’m not surprised. It is that kind of wishful ignorance that results in politicians selling for several generations now the fantasy that the state can provide lots of services and benefits without having to fully pay for them and rack up a gargantuan debt. Or that you can have a pension age of 65 with a life expectancy of 80 years.
People would rather have politicians tell them a lie they want to hear than the awkward truth.
And so we are where we are.
So, Liechtenstein and Monaco in dire straights, then?
Except it doesn’t. Indeed the UCL paper which Migration Watch took apart, noted only that WRT immigrants with professional qualification did benefit the economy, the rest was a far far greater burden.
So sorry. Migration Watch are a respected and long standing institution.
Stewart——–You are beginning to be like a hammer that sees everything as a nail. We know what you are saying. There is truth in it. But there are other truths as well that need to be balanced, and I do not think we have that balance correct in this country. Infact we are making a total mess of it, but the scary thing is that it is deliberate as our politicians pander to the UN and WEF rather than to the people who vote for them.
“In the short term, all else remaining equal, there would be a drop in population as total immigration exceeds population growth and has for many years now.”
England, bar Malta, is now the most densely populated country in Europe. Less people would be a positive benefit.
Maybe so, in some ways. Will it lead to more economic growth or less? Less.
That’s a price I may be persuaded to pay. What I’m not being persuaded at least by any arguments on here is that it’s not going to less economic growth. I try to avoid irrational wishful thinking.
I never understand these whataboutery lines of argument. Nothing exists in isolation and there will always be consequences of any particular action. So what? You adjust. Its an argument for inertia.
Politicians, bankers, industrialists, imply that immigration is good because it increases GDP. (Gross domestic product).
Growth is good! That was one of our mantras in the 1990s. So increasing GDP is good, right?
Wrong! GDP growth is NOT the same as productivity growth which is more likely to enrich a nation. Productivity per capita is not talked about much by UK politicians! Maybe because our productivity per capita performance is dreadful.
Generally, for mature economies if you set a high skills bar for immigrants then productivity per capita may increase but if, like the UK which has lost control of immigration, resulting in high levels of unskilled people then productivity will likely fall.
That’s very very funny, regardless, don’t give up the day job. These figures will only have got worse, by a large degree.
https://www.migrationwatchuk.org/briefing-paper/347
“Response to UCL paper on the fiscal effects of immigration to the UK30 December, 2014
Summary
Overall cost of migration
1. Between 1995 and 2011 the fiscal cost of migrants in the UK was at least £115 billion and possibly as much as £160 billion according to a report from the Centre for Research and Analysis of Migration headed by Professor Christian Dustmann at University College, London. The report found that migrants in the UK were a fiscal cost in every year examined.[1]
Contribution of ‘recent migrants’
2. The report claims that migrants who had arrived in the UK since 2000 had made positive contributions throughout the period from 2001 to 2011. This does not appear to be correct, the figures in the paper show that the contribution from these recent migrants was negative in each year after 2008.
Contribution of ‘recent A10 migrants’
3. The authors also highlighted a finding that between 2001 and 2011 recent migrants from Eastern Europe had made a net contribution of £5bn. While this correctly reports their most optimistic finding, their calculations in four alternative scenarios were all lower. One of these alone was enough to reduce the contribution to as little as £0.066bn – a sum within the margin of error of such calculations.”
And then there’s the cost of “asylum seekers” (aka “economic migrants”. If you think immigration benefits the country financially, you are bonkers. Every 250,000 net in requires the equivalent extra public services to be provided as a small city. And as we all know, our public services are laughable as it is.
A few years ago when I worked at the DWP a couple with two children taking in their housing costs, free prescriptions, school meals, child benefits and neither parent working would easily have a PAYE equivalent income of £25K.
Add on some Disability Benefits – very easy with a bit of ADHD – and before you know it the figures are £35 k plus.
The most insulting figure I came across was a PAYE equivalent of approx £60k but similar figures were common.
Oh and Child Benefits used to be “exported” to Poland at £1 million per month.
God knows how much went to our Indian subcontinent “ex-pats” who went home to “retire.”
The vast majority of the British public haven’t got a bloody clue about our benefits system.
I’m not sure immigrants get most of the benefits or even a large proportion of them. I’d like to see some evidence of that, a breakdown of who gets benefits perhaps.
But either way, perhaps we can agree that benefits to working age people should be scrapped altogether?
I understand your point, while not wholeheartedly agreeing and, if that was how this discussion was being framed then I would be up for the debate.
However, this is not how the discussion is proceeding.Those with any reservations are painted as bigots, eg. by that odious intellectual fly-weight Owen Jones.
Your argument seems to be that if we welcome everyone who makes the attempt to come then we will automatically benefit. This overlooks entirely reasonable observations that we should perhaps be able to control the rate of arrival, and also more strictly favour the legal route, allowing us to vet the newcomers rather than ferrying in everyone who can climb into a boat.
We have finite capacity, which won’t change without serious investment. We aren’t creating the infrastructure to help absorb the numbers coming here and they arguably have a downward pressure on the wages of those already here, perhaps benefiting industry but certainly not the general population
Not exactly. I am pointing out that there are benefits to immigration without in any way denying the many drawbacks. And that the public may not be prepared for the consequences of cutting back severely on immigration.
And when I see people on here try to argue that there would be no negative consequences it suggests.to.me I’m right.
Which doesn’t mean I’m against cutting back sharply on immigration.. In fact, I really hope they do if for no other reason than it’ll prove.my point.
Also because I agree there is too much. But it will cause economic problems for sure. A price will need to be paid is all.
For some reason just saying that triggers people on here.
Hi Stewart – sorry to add to your workload on this thread! Immigration / rising populations add to gdp and therefore this is commonly described by politicians as economic growth (and will keep us in the G7). This is true. But that is a long, long way from saying we are better off as a country, particularly native Brits. That’s all this argument is about. Your second point is a question about whether Brits will accept low/no immigration if fruit and social care goes up in price. They will if they get a proper cost/benefit analysis shown to them on the BBC. But will that happen since the BBC answers to the new world order and are seeking to destroy this country? Not likely.
I did the work. I made no reference to immigrants. If you want evidence of benefits by status or ethnicity in whatever metric you choose put in an FOI. Otherwise STFU and read my following posts on this subject.
I did the fuckin work so don’t dare contradict me without evidence.
It would be nice to think, having witnessed the hundreds of thousands marching in support of Islamist terrorism on the streets of London, the pro-immigration/multi-multiculturalism Establishment would be considering how to:
a) force integration
b) deport foreign terrorist sympathisers
c) strengthen our borders
d) reduce immigration from the “problem” areas (middle east, Muslim Africa, Muslim Asia)
But they’re doing none of them. Instead, they’re preparing for the (inevitable) terrorist atrocities in the UK.
Over the past 50 years, and in particular the last 20, they seem to have been determined to prove Enoch right.
I don’t think you can ‘force integration’, that has to be something coming from the one you want to integrate and I get a feeling that this is not what ‘they’ want at all. They see us as weak, rotten, corrupt, unbelievers…basically infidels. They want US to integrate into THEIR culture.
”They want US to integrate into THEIR culture”. Not integrate, submit to.
Well Trump tried to put a stop to people coming from dangerous places but that was turned into a “Muslim Ban” by the Left
To me this is very clear. The actual will to stop illegal immigration is entirely lacking on both sides of the channel. It’s all fogged by things like the ECHR’s rulings and so on and the repeated attempts to undermine any meaningful constructive solution by the left. Honestly, if I was an immigrant, I’d want to go to Rwanda because it’s warm, get’s loads of aid, has brand new roads and infrastructure and looks wonderful. Anyway, apparently, we pay the French to stop the boats but the boats keep coming. So, what are they doing with the money we pay them? Having extended breakfasts of coffee and croissants? And then there is the role of the RNLI and Royal Navy who are now mere taxi drivers. There’s no border at all. If you’re a young religious fanatic or a drug dealer and you’re on the run what better place to go than the UK where you get motored back to the mainland, given a free home, a phone, money, dental and medical care. Result!
And day after day, the boats keep coming and day after day they have to find new places – hotels, army camps, barges etc – to put them. There is talk that these men are mainly military age, are fit, seemingly unaffected by whatever trauma they are meant to have gone through, and then they are put in places where they are only going to get bored and frustrated. And then what? What does our government plan to do with hosts of bored young men, some of whom are no doubt used to violence judging by the countries they apparently come from. So far, my neck of the woods is fairly immigrant free but I am sure they’ll come one day and all the liberals will be trying to welcome them in as if they are here to be helpful, to integrate, to adopt British ways, have tea at 3, learn the rules of cricket and so on. I very much doubt it.
Personally, I am not a fan of what is happening to my country. I don’t wish to see it become a caliphate. It is a Christian country. If they want to change it to suit these incomers, I will be one of those resisting it. We had the bloody Normans come over here in 1066 and they created the establishment that still rules today, we don’t need another invasion, things don’t generally go well. I believe that the next GE will be fought on immigration even though it would be good to clear the air of CBDCs, digital IDs, and all the other Agenda 2030 stuff but of course that is just us conspiracy theorists who get concerned about that!
”It is a Christian country.” Not any more. Didn’t you see the recent demonstrations of hundreds of thousands of Islamists in the major cities? And the disrespect for what is arguably the epicentre of Britishness, the Cenotaph. The country and culture that you speak of and that we value is gone for good, in part because of the tolerance which it represented. And which the newcomers do not share.
We are being shat on while we sit watching soap opera’s
It is still largely a white Christian country even though those who identify as Christians has diminished. Yes, there are huge demonstrations by islamists but the Muslim religion still only represents 6.5% of the country. Outside of some of the major conurbations where the mix is more diluted, the old country still exists in many places and I don’t agree that it is gone.
Wake up, demographics are destiny. Compare birth rates.
I’m perfectly awake but thanks for the reminder! I was talking about the population and country as it is now. Anything can happen in the intervening years, in fact all sorts of things are already happening. I guess I’m not so pessimistic as you.
I commend your post Aethelred and agree wuth much of it although your leniency towards the invaders could do with hardening. However, we are definitely NOT “conspiracy theorists.”
Conspiracy Realists.
When you take a glass of orange juice and continually pour pineapple juice into it you eventually don’t have orange juice anymore. Ok but orange juice and pineapple juice are both quite nice anyway. —–But what happens if what you are pouring into the orange juice is not something very nice?
I would identify three objections to significant levels of immigration:
1
Cultural. The idea of multiculturalism always implied ghettos and when the political class decided they wanted unlimited numbers a clash was inevitable. White, western, liberal democracy (whatever that used to be) would not survive the primitive adherence to a religion practiced by societies unchanged for centuries from whom the immigrants were to come.
2
Quality. If those coming were to work on high skill jobs which we did not have enough of then their contribution would exceed their marginal cost to the existing population. As work permit temporary workers they would not impose long lasting pressure on infrastructure. Under the general heading of “quality” I would keep out those who teach hatred and cultural isolation.
3
Numbers. The numbers are important because of the impact they have. As has been said of military affairs, quantity can become a quality all of its own.
Large numbers create difficulty with intergration. I know the political class has not wanted integration since the boom they created in numbers in the 1980s. They proclaimed the benefits of multi-culturalism. Integration and multi-culturalism are competing values and it is unlikely we could ever have both and we certainly don’t.
Large numbers disrupt the existing workforce. They suppress wages through competition for jobs. This has been especially significant during decades when the UK economy de-industrialised (to meet the Net Zero fantasy) and so many Brits lost their jobs abd their skills were no longer in demand.
Numbers put extreme pressure on infrastructure. As they mostly arrive with little or no money, earn little and remit any spare cash to families “back home”, the new immigrants make negligible provision to current spending and nothing at all to the capital cost of infrastructure and housing. Either the pre-existing population has to pay for more infrastructure capacity or (as more often in the UK) it is not built so we pay through queueing. We queue to use facilities we paid for years ago but which we now have to share with millions of others.
Of course, very little of this affects the political class. The new elite which Matt Goodwin so convincingly desribes know and care litte about all this. They look out from their sheltered living space using the blinkers that are issued when they first signed up and do not notice or care about complaints or opinions or votes of the little people.
Can relative peace continue as the immigration numbers continue so high, house building drops with the impoverished economy and frustrations multiply. All it needs is a minor war between long term adversaries to ignite stteet violence.
Regarding your last paragraph, I’d say the UK is looking at the dire situation Sweden is in if things don’t change soon, and the UK police have demonstrated who’s side they’re on. Nowadays you get warned not to be racist and told to put your England flag away out of sight at a demonstration made up of mostly Muslims, just to give one example. It’s not even just the newcomers that we need to worry about, as per the Swedish example it’s also the second and third generation immigrants. Soon the UK will also have ‘no go zones’, whole ghettos under mob rule, as opposed to adhering to the law of the land, where parallel societies are very much the reality. Germany and France are the stepping stones to get to the Swedish situation.
Meanwhile in the UK it would appear the government has been spoiling the immigrants so much that they’ve gotten used to a certain standard of living and treatment. It makes you wonder what the living conditions are like back home if this is how they react to getting moved out of their hotel;
”After allowing their island to be overrun by mass migration, the supposedly conservative British government, now led by Rishi Sunak, is trying hard to find a way to deal with the situation – both in terms of public safety and also in terms of financial impact on its society of housing these people – that rose to 7 million pounds per day.
Let’s be honest: once it’s established that you’ll give migrants nice free hotels, food and welfare checks – the whole world will flock to your doorstep.
So the new measures the government is trying to implement are purposefully harsher than the previous ones.
But where is the limit? Where does it becomes an infringement of people’s human rights? The discussion has inflamed the country.
One of the not-so-successful solutions is using the Barge Bibby Stockholm as a cost-saving housing for people awaiting to have their asylum claims checked.
A lot has gone wrong so far, and it seems to be getting worse.
A 23-year-old Nigerian man tried to kill himself in an Essex hotel car park upon hearing that he was due to be transferred on to the Bibby Stockholm barge.
He was airlifted to hospital and placed on life support.”
https://www.thegatewaypundit.com/2023/10/uk-migration-chaos-nigerian-asylum-seeker-reportedly-tries/
Excellent synopsis
At Terminal 5 Heathrow last week at least 9 put of 10 workers were not white
Without immigration everything would grind to a halt very quickly
whitey can be a bit lazy
give him enough money to have tattoos and an XL Bully and he will happily enjoy a life of leisure.
Getting 5 million off the social is the challenge
You have assumed the non white workers were all hired legitimately and both they and their companies paid tax.
Where I live there is a dual system where non whites can buy & sell properties outside the law/LandRegistry/Council Tax/Occupancy levels/ Gas safety etc, etc and fill both the property and their coffers at the same time.
They even have their own ‘Banks’ where they pay in cash with no questions asked.
“Whitey can be a bit lazy”
UK unemployment rates, second quarter 2023:
White: 3.5%
Mixed: 6%
Chinese: 6.1%
Indian: 7.1%
Black: 7.2%
Pakistani: 9.7%
Bangladeshi: 10.3%