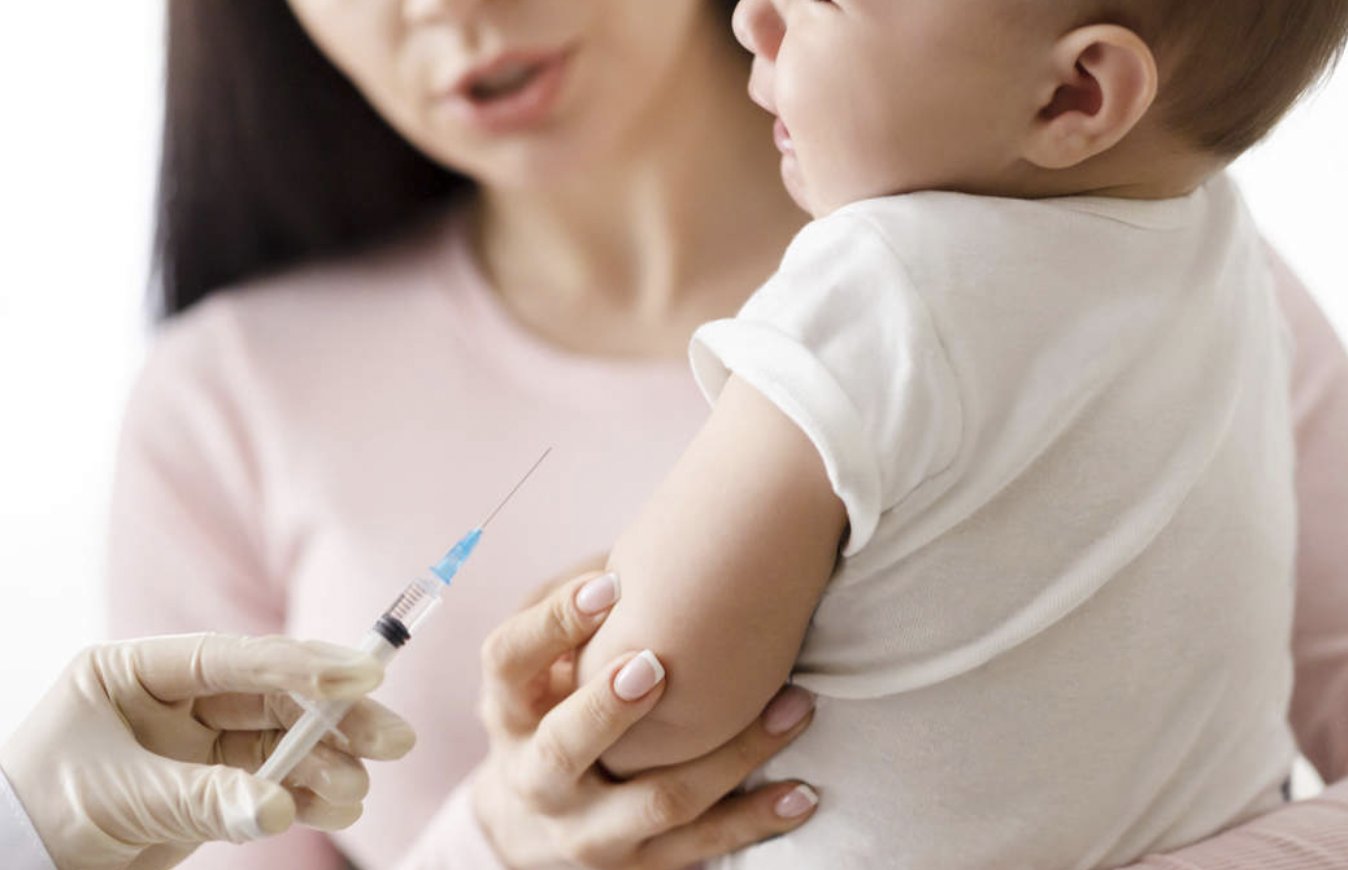
It’s been less than a week since reports suggested Pfizer was preparing to seek approval from U.S. and European medicines agencies for its vaccine in 5-11 year-olds. The pharmaceutical is now seeking authorisation for the vaccination of American babies as young as six months this winter. MailOnline has the story.
In a move likely to cause international controversy, the company intends to apply for authorisation to immunise American infants within the next two months.
The timeline will depend on the findings of in-house trials looking into whether the vaccines are safe and effective in youngsters aged six months to five years.
Frank D’Amelio, Chief Financial Officer at Pfizer, told an industry conference yesterday that the firm plans to “go file” by November, the Financial Times reports.
“We would expect to have… data for children between the ages of six months and five years old that we would file with the FDA,” D’Amelio said at the Morgan Stanley Global Healthcare Conference. “I’ll call it in the weeks shortly thereafter the filing of the data for the five to 11 year-olds.”
Pfizer was already planning to seek approval from the Food and Drug Administration (FDA) for the jabs to be given in children aged five to 11 by October.
But the latest comments confirm the firm’s intention to work its way down much younger age groups. They will be given a lower dose than adults. …
Scott Gottlieb, who headed the FDA under former President Donald Trump and now sits on the board of directors at Pfizer, says that the emergency use approval process for vaccinating young children could be done in a matter of weeks.
Gottlieb says the pharmaceutical giant is expected to file the paperwork with the federal government requesting authorisation to vaccinate kids as early as September.
Worth reading in full.












To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
“gender-critical”
=
People who correctly identify that there are two immutable sexes determined by fate/God at inception, and that “gender” is a nonsense concept outside of grammar. Probably 99% of world population, and historically probably 99.9%, share this view. It’s the others who are fringe loonies, not us.
Well said tof. I heartily object to being criticised and labelled for being ‘normal.’
In the German navy, their used to be a saying
Ein Elfer mit einem Schlauch ist gefährlicher als ein Affe mit einem Maschinengewehr.
[A sailor with a deck-washing hose is more dangerous than a monkey with a machine gun]
They may be loonies. But they’ve been handed a very real power to harm other people.
You should never give a monkey a machine gun, it could end in a massacre.
http://www.huffingtonpost.co.uk/entery/borat-is-back-to-give-a-w_n_71388
What, may I ask, is self-identified gender?
The fact that this sort of sentence can now be written perfectly seriously and widely accepted without question is a testament to the level of collective insanity that is afflicting our society.
On current trajectory, within a few years, we will be reading and taking seriously sentences like “biological species takes precedence over self-identified species.” Sounds crazy now, but give it time.
It is positively impossible that men can become women. The opposite is not an idea which can legitimately be challenged, it’s an absurd article of faith which has to be rejected whenever its proponents try force it onto the infidels. They have every right to believe in anything. And everybody else has the same right to ignore whatever they happen to believe in. Reality doesn’t change by altering someone’s description of it.
https://www.conservativewoman.co.uk/mask-mania-claims-a-little-boys-life/
This will bring tears. A little lad murdered by the enforcement of masks.
The Groan rightly gets a good slapping.
Short version: 11 month old boy suffocated by his own mucus after professional child-minder forced mask back onto his face.
Gruelling.
Something for some context:
https://news.sky.com/story/the-virus-is-still-evolving-at-an-incredible-rate-how-widespread-is-covid-now-and-how-many-people-are-dying-with-it-13059424?utm_source=pocket-newtab-en-gb
Some highlights:
Prof Griffin says the UK is “not suppressing prevalence”, which means “we’ll continue to see those waves”.
“Anti-vax sentiment clearly got highlighted during COVID – but I think most people know that those vaccines really work and that in a world without them, it would be Christmas 2021 again.”
Christmas 2021 was a year after mass vaccination had started in the UK. So, without the marvellous Covaxxes, we’d be right in the same situation as if we had had them?
“So children turning five after September 2022 have to wait until they’re in their 60s to have a vaccine, unless they become clinically vulnerable,” Prof Griffin says.
“The idea that repeated infections are a preferable means of generating population immunity to vaccines, especially in children, is a dangerous nonsense.”
“COVID is a spectrum and long COVID is only one end of that,” Dr Duncan says.
Dr Duncan says that we “urgently need multi-layered public health protections”, including seasonal vaccines and ventilation systems for cleaner indoor air to “stop people continuously re-infected with constantly evolving new variants”.
And then, it obviously mentions everything else which absolutely has to be mentioned, including social inequalities, MMR vaccines and the dangers of smoking.
https://www.conservativewoman.co.uk/i-am-so-angry-with-the-morons-who-let-the-chemical-attacker-stay-here/
Laura Perrins letting rip on those guilty of allowing the acid attacker to remain in this country: an illegal immigrant, a three times failed asylum seeker, a convicted sex offender and now all but a murderer.
Christians don’t commit murder. These Next Tuesday mussies do.
Stonewall have infested the public sector like a nasty virus
With NO public mandate whatsoever.