A new peer-reviewed study by Dr Pierre Kory and colleagues on Ivermectin has been published in the American Journal of Therapeutics. Entitled “Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19“, it provides a new authoritative overview of the evidence to date and calls for the widely available drug to be “globally and systematically deployed in the prevention and treatment of COVID-19”.
The study summarises the impressive evidence base for the use of Ivermectin.
1. Since 2012, multiple in vitro studies have demonstrated that Ivermectin inhibits the replication of many viruses, including influenza, Zika, Dengue, and others.
2. Ivermectin inhibits SARS-CoV-2 replication and binding to host tissue through several observed and proposed mechanisms.
3. Ivermectin has potent anti-inflammatory properties with in vitro data demonstrating profound inhibition of both cytokine production and transcription of nuclear factor-κB (NF-κB), the most potent mediator of inflammation.
4. Ivermectin significantly diminishes viral load and protects against organ damage in multiple animal models when infected with SARS-CoV-2 or similar coronaviruses.
5. Ivermectin prevents transmission and development of COVID-19 disease in those exposed to infected patients.
6. Ivermectin hastens recovery and prevents deterioration in patients with mild to moderate disease treated early after symptoms.
7. Ivermectin hastens recovery and avoidance of ICU admission and death in hospitalised patients.
8. Ivermectin reduces mortality in critically ill patients with COVID-19.
9. Ivermectin leads to temporally associated reductions in case fatality rates in regions after ivermectin distribution campaigns.
10. The safety, availability, and cost of ivermectin are nearly unparalleled given its low incidence of important drug interactions along with only mild and rare side effects observed in almost 40 years of use and billions of doses administered.
11. The World Health Organisation has long included ivermectin on its “List of Essential Medicines.”
The quality of the evidence for Ivermectin has been challenged, leading many countries including the U.K. and U.S. not to recommend its use for COVID-19. The study takes this criticism head-on.
Although a subset of trials are of an observational design, it must be recognised that in the case of ivermectin (1) half of the trials used a randomised controlled trial design (12 of the 24 reviewed above) and (2) observational and randomised trial designs reach equivalent conclusions on average as reported in a large Cochrane review of the topic from 2014. In particular, OCTs that use propensity-matching techniques (as in the Rajter study from Florida) find near identical conclusions to later-conducted RCTs in many different disease states, including coronary syndromes, critical illness, and surgery. Similarly, as evidenced in the prophylaxis and treatment trial meta-analyses as well as the summary trials table, the entirety of the benefits found in both OCT and RCT trial designs aligns in both direction and magnitude of benefit. Such a consistency of benefit among numerous trials of varying sizes designs from multiple different countries and centres around the world is unique and provides strong, additional support.
A hint of the politics around Ivermectin can be gleaned in the discussion section, where the authors wonder how much more evidence a cheap, safe drug like Ivermectin needs in an international emergency before it can be approved.
The continued challenges faced by health care providers in deciding on appropriate therapeutic interventions in patients with COVID-19 would be greatly eased if more updated and commensurate evidence-based guidance came from the leading governmental health care agencies. Currently, in the United States, the treatment guidelines for COVID-19 are issued by the National Institutes of Health. Their most recent recommendation on the use of ivermectin in patients with COVID-19 was last updated on February 11th, 2021, where they found that “there was insufficient evidence to recommend for or against ivermectin in COVID-19”. For a more definitive recommendation to be issued by major leading public health agencies (PHA), it is apparent that even more data on both the quality and quantity of trials are needed, even during a global health care emergency, and in consideration of a safe, oral, low-cost, widely available and deployable intervention such as ivermectin.
The authors add that further evidence is on its way and express an earnest hope that “if the above benefits in clinical outcomes continue to be reported in the remaining trials … this almost doubling of the current supportive evidence base would merit a recommendation for use by the WHO, NIH, and other PHAs”.
The study includes two striking graphs, from Peru and Uruguay, illustrating the strong association of Ivermectin use with reduction in Covid infections and deaths.
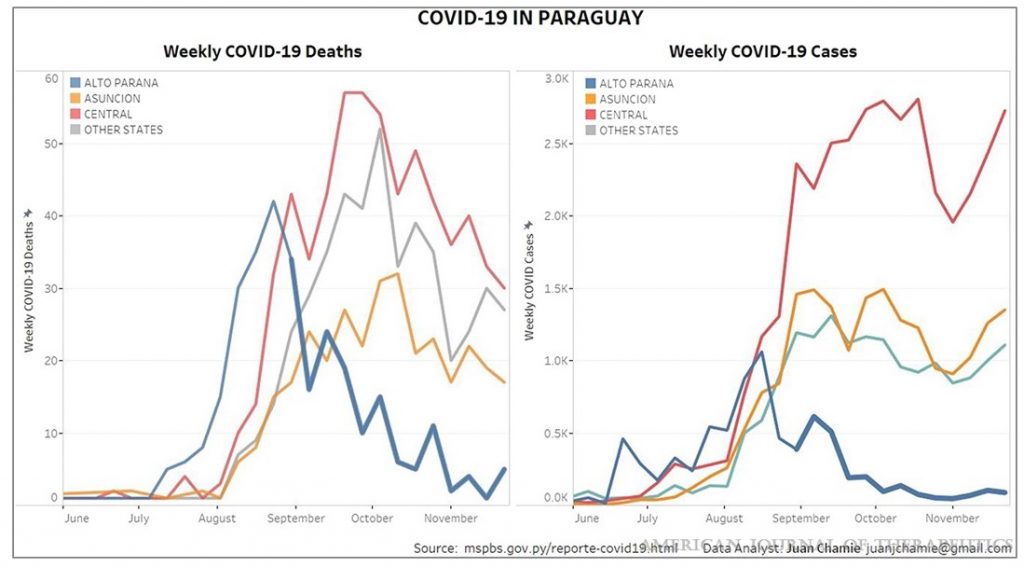
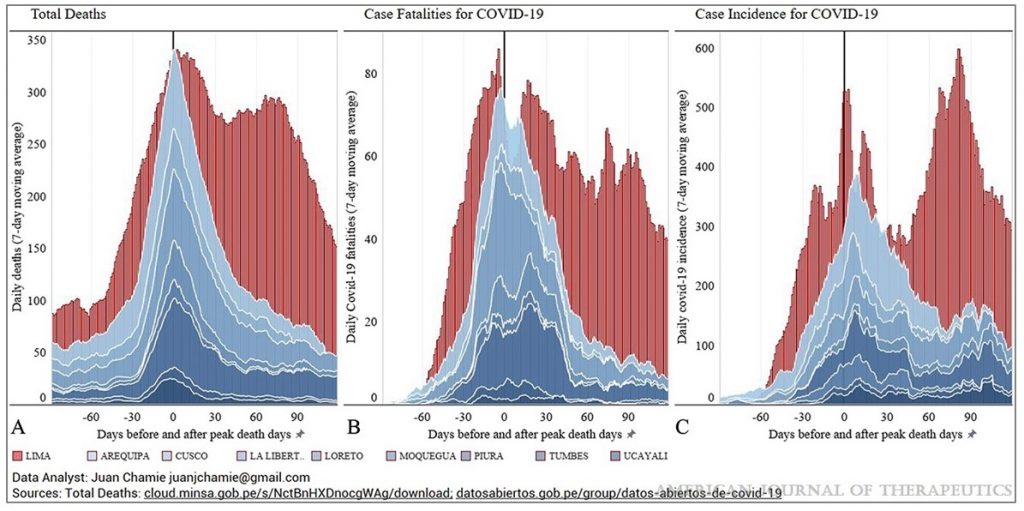
Considering how quickly vaccines using novel technology with unproven long-term safety records have been authorised for use, the failure to approve a known safe drug with considerable evidence to suggest clinical benefit is almost inexplicable. Some have suggested the resistance has been motivated by a wish among pharmaceutical companies to prioritise more profitable medicines like the vaccines. If so, this is a criminal example of putting profits before people.
It is good to see this study make it to publication. (There are rumours that some journals rejected it, not for any technical reason but because it would be controversial, possibly with their sponsors.) It is to be hoped there will be many more such studies, and a sea-change in regulators’ attitudes to repurposed medicines like Ivermectin.













To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.