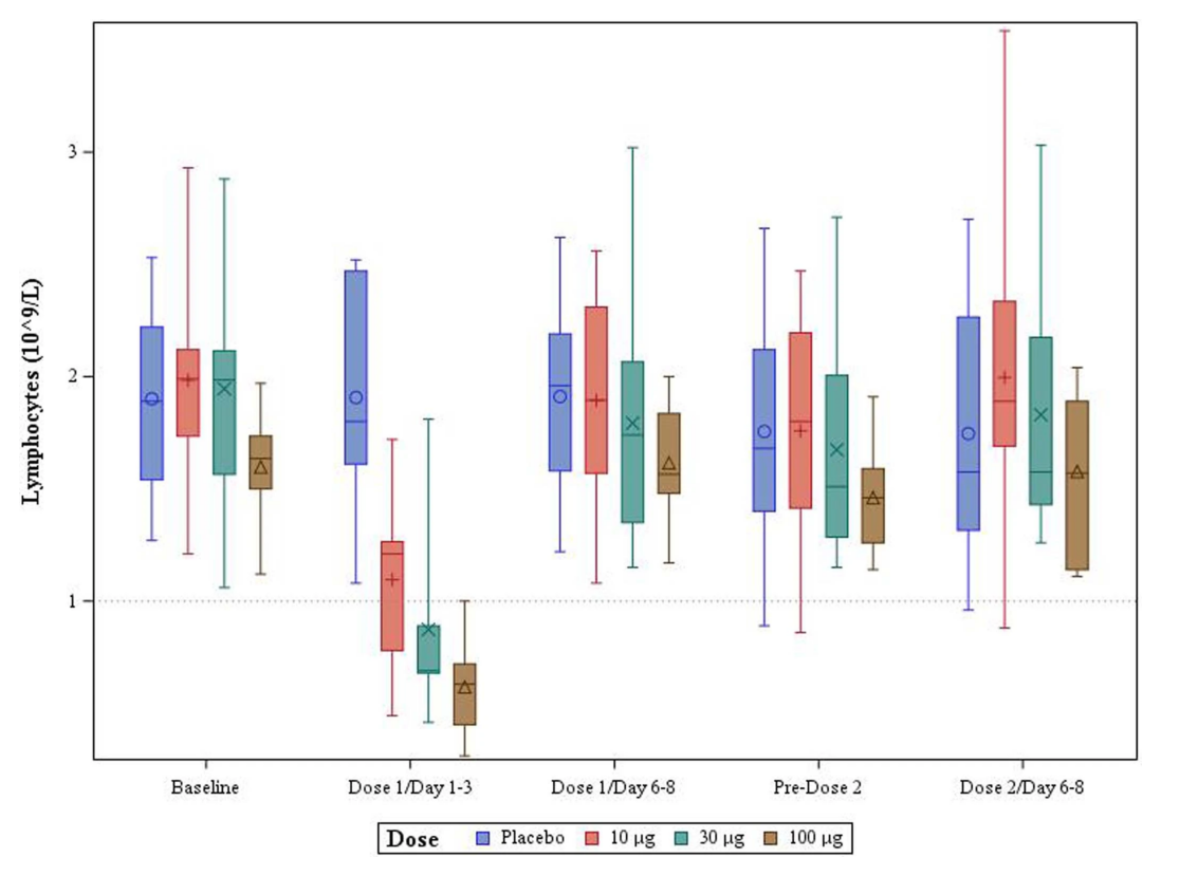
Since December 2020 the UK public has been unwittingly involved in a huge experiment that will reveal the clinical effects, both positive and negative, of the Pfizer/BioNTech (PF)1 and Oxford University/AstraZeneca (AZ)2 COVID-19 vaccines. Despite the absence of thorough animal trials of these products and lack of any human data beyond Phase 2 trials lasting a matter of months, they have been approved by the MHRA and are now being deployed in a programme of mass vaccination. As part of its statutory functions, as well as its legal and moral duty, the MHRA is responsible for monitoring the effects of these vaccines to ensure that their benefits to patients outweigh any risks.3 There are a number of reasons for anticipating that these particular vaccines pose more risk to the general public than traditional vaccines. Traditional vaccines contain products against which the immune system directly generates antibodies. These may, for instance, be inactivated forms of an otherwise harmful virus. Whatever their nature, they are not intimately associated with living human cells. In contrast the PF and AZ vaccines operate by hijacking the protein-producing machinery of human cells causing them to produce and display the spike protein of the SARS-CoV-2 virus. The immune system then raises antibodies against these proteins, thus providing protection against the real virus. However, the workings of the ...