In a recent article in the BMJ, a whistle-blower exposed serious problems she had observed first-hand in the Pfizer vaccine trial in Texas.
A regional director who was employed at the research organisation Ventavia Research Group has told the BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial. Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding. After repeatedly notifying Ventavia of these problems, the regional director, Brook Jackson, emailed a complaint to the US Food and Drug Administration (FDA). Ventavia fired her later the same day. Jackson has provided the BMJ with dozens of internal company documents, photos, audio recordings, and emails.
Another Ventavia employee said of the data the company generated for the Pfizer trial: “I don’t think it was good clean data. It’s a crazy mess.”
The six-month trial results for the Pfizer vaccine have now been published in the New England Journal of Medicine. These findings, the researchers note, “contributed to the full approval of BNT162b2 [the Pfizer vaccine] in the United States”. A close inspection of the study, however, reveals a number of problems that raise serious questions about the reliability of its findings, as well as about the safety of the vaccine.
Here is the graph of cumulative incidence for the two trial arms, vaccine and placebo, over the six months of the study period, showing how the symptomatic Covid PCR-positives added up following receipt of the first dose.
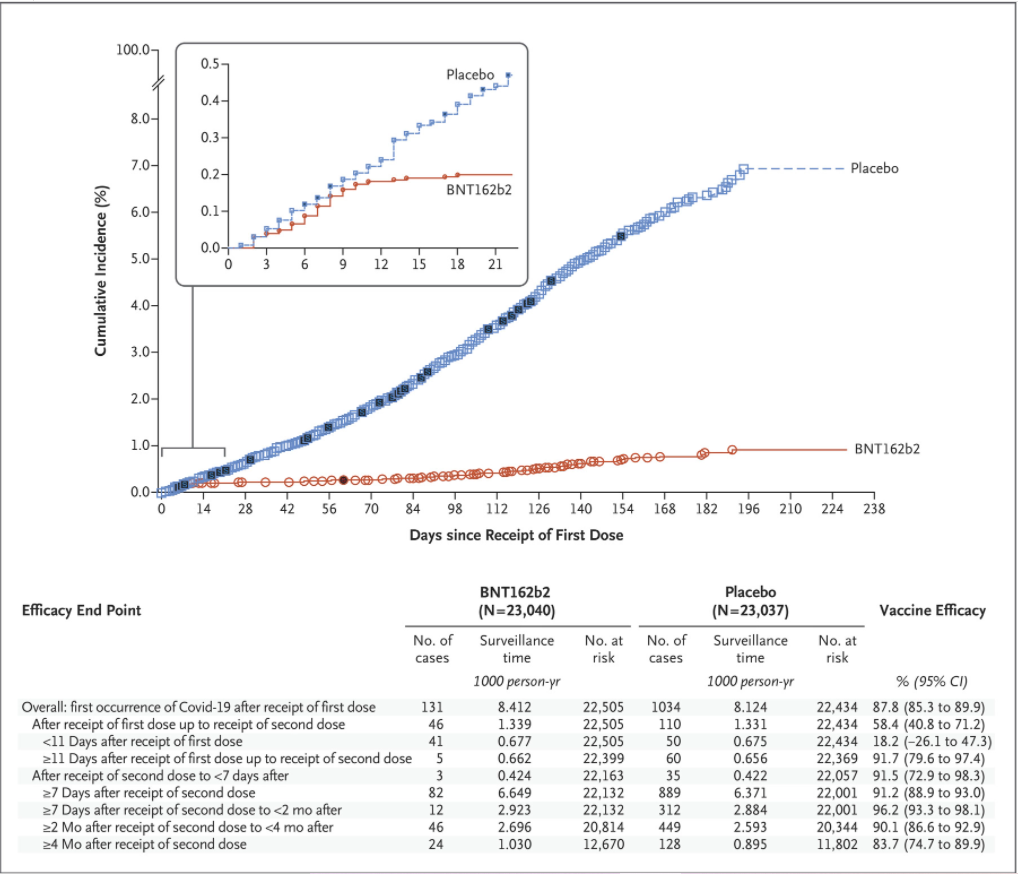
The first curious feature is that vaccine efficacy abruptly kicks in on day 12 after the first dose. Prior to that it’s near zero (18.2%), as expected, but then all of a sudden incidence in the vaccinated arm grinds almost to a halt and the vaccine efficacy leaps to 91.7% and stays there. Second doses were given at 21 days, but there is no sign of (or room for) an uplift in efficacy at that point. This high efficacy of a first dose at day 12 is in conflict with the results of a real-world mass observational study in Israel covering a similar time period (December 20th to February 1st), which found vaccine effectiveness against symptomatic Covid for days 14-20 after the first dose to be just 57%.
The trial study authors note that vaccine efficacy drops to 83.7% after 4-6 months, a fall of around 3% per month. Where, though, is the sharper decline seen in other studies, such as those from Sweden and Qatar? Is it because the study period ends on March 13th 2021, so pre-dates not only Delta but also Alpha in the countries where the trial was being carried out (mainly USA, Brazil and Argentina)? It’s worth noting that during the observational study period Israel did have a high prevalence of Alpha. If this is part of the reason for the discrepancy, it calls into question how applicable the findings are to the present context where Delta dominates.
Unfortunately, the trial can no longer help to answer these questions as, the authors explain, “most participants who initially received placebo have now been immunised with BNT162b2, ending the placebo-controlled period of the trial”.
Another question is why the severe cases (marked in black on the chart above) are not evenly distributed. Why do they appear in clumps with gaps, when the other symptomatic infections increase evenly? Also, there is only one severe case in the vaccine arm and 30 in the placebo arm (giving a reported efficacy against severe disease of 96.7%). Yet three of the severe cases in the placebo arm occurred in the first 12 days after the first dose, before the vaccine is supposed to start working and when there is no difference in the numbers of non-severe cases. Why then are there no severe cases in the vaccine arm in that period? Why, also, is the ratio of deaths in the two arms one-to-two (vaccine to placebo) but the ratio of severe cases one-to-30?
Some of the figures for people who dropped out (see below) are also odd.
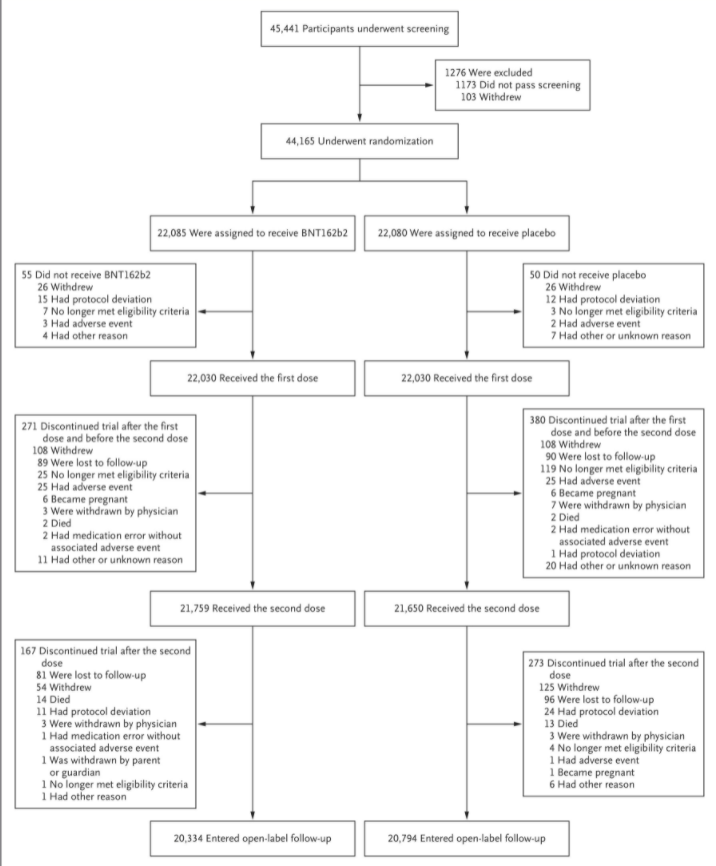
Note that in each arm, prior to one dose, exactly 26 people withdrew, and after one dose, exactly 108 people withdrew and 25 had an adverse event (odd considering the placebo was saline). Six became pregnant and two died in each arm, while 89 (vaccine) and 90 (placebo) were “lost to follow up”. A professor of statistics wryly observed to me it was “as if they have gone to some trouble to show how remarkably well randomised the trial was”.
Someone sent me this handy graphic showing all the deaths recorded in the study.
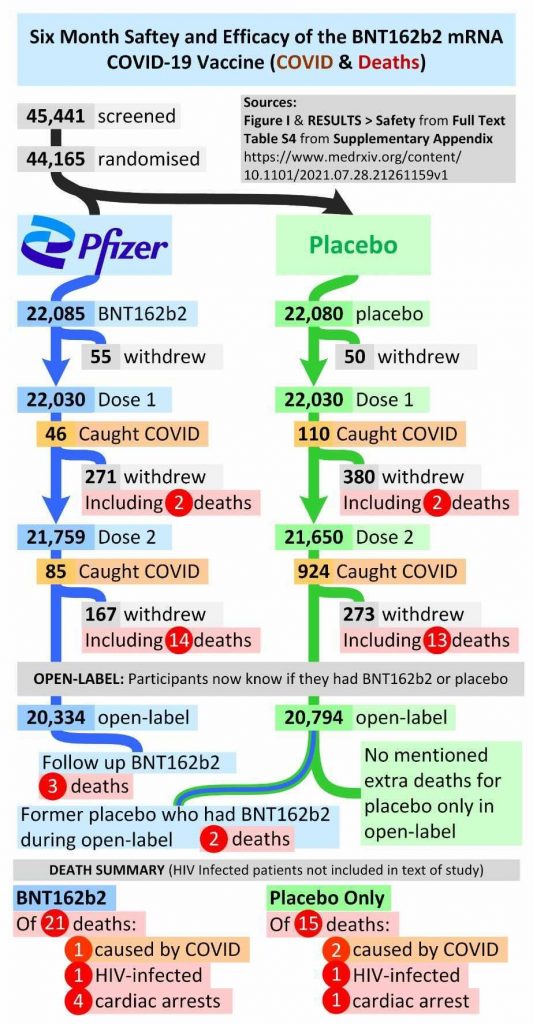
Notably there are 21 deaths from all causes among the vaccinated and 15 among the unvaccinated – though if we exclude the five from the period after the unblinding when most of the placebo group received the vaccine (and no more deaths in the placebo group were recorded) it’s 16 vs 15. Alex Berenson has spotted a report where Pfizer state it was actually 21 vs 17 by the end of the blinded study period on March 13th, raising questions of why there seems to be ambiguity on this important data point. Either way, there are more deaths from all causes in the vaccine arm than the placebo arm, and significantly, perhaps, one of the big differences by cause is there are four versus one heart attacks. Despite this, the researchers claim: “Causes of death were balanced between BNT162b2 and placebo groups.”
Inevitably, though: “None of these deaths were considered to be related to BNT162b2 by the investigators.” Also: “No cases of myocarditis were noted.”
The data on natural immunity is confusing. The authors state that: “Participants with a history of COVID-19 were excluded, although evidence of current or previous SARS-CoV-2 infection on laboratory testing of trial-obtained samples was not an exclusion criterion.” This suggests they excluded those who had had symptomatic Covid before, but not those who’d tested positive asymptomatically. However, then they state: “Nine cases of COVID-19 were observed among participants with previous serologically defined natural infection: two cases were observed among the vaccine recipients and seven among the placebo recipients.”
How can there be a significant number of people in the trial with SARS-CoV-2 antibodies if they excluded anyone with a history of COVID-19 – assuming that you don’t get antibodies from an infection so mild it produces no symptoms? This needs to be cleared up, not least because the authors claim their data “support the current practice of immunising without screening for evidence of previous infection”.
With so many questions and concerns about the trial and its data, it’s hard to know how it can be trusted, despite supposedly being the gold standard of evidence.
That’s certainly the feeling of Surya Arby, a consultant in France, whose BMJ ‘rapid response‘ to the whistle-blower report remarks: “It’s hard to understand how we can trust the safety data provided by Pfizer.” Surya notes that “the official package insert approved by the FDA for Comirnaty [Pfizer] states that acute allergic reactions (including anaphylaxis) have been reported only in post-marketing surveillance (including EUA)”. However, “in the real world, the observed rate of acute allergic reactions is close to 2% (1.95% [1.79%-2.13%]) and the observed rate of anaphylaxis is close to 1/3,700 for mRNA COVID-19 vaccines (Pfizer 0.027% [0.011%-0.056%])”.
Such a rate is “impossible to miss,” he says, “in a cohort of 21,700 vaccinated individuals in a clinical trial”.
Indeed, yet ‘miss’ it they did.










To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
No.
Yes. I trust them to harm.
What is Latin for “Do Some Harm”?
Vaxxinate! Vaxxinate! Vaxxinate!
Veni Vidi Fauci
Try reading this, it answers a lot of questions about mRNA “vaccines” , but not for the Faint Hearted …..
https://notrickszone.com/2021/11/17/doomsday-vaccine-german-physician-corona-vaccines-a-programmed-self-destruction-of-the-body/
Tes. It has been a very profitable exercise for Pfizer, as planned. It has also gutted the concept of voluntary clinical trials.
…
I read that the data was “manipulated” from trials forced on them, post EUA decision, because of the concern over the effects on pregnant women – admittedly not by Pfizer in the first instance but….
https://theexpose.uk/2021/11/07/cdc-scientists-admit-they-did-manipulate-study-data-to-show-the-covid-19-vaccines-are-safe-for-pregnant-women/
Depends what you mean by trusted. It must have been good for their bank balance, e.g., so I’d believe in that, but not much else.
Can anything we hear about the NHS be trusted?
Yesterday I had a one hour meeting with a physio nurse during which she pocked and prodded before offering advice about exercise and whatnot.
She also asked me to arrange a follow up hour session in 3-4 weeks at dept. reception on the away out (thus bypassing the bureaucracy).
On checking in at the Mycare Patient Information Portal I find that I also have an appointment for a Transthoracic Echo scan (wtf?) in less than three weeks.
The NHS is supposedly months adrift in all manner of treatments and testing, I have already experienced getting put at the head of the queue by a Consultant but the two further appointments were at the behest of a lowly physio Nurse.
Not that I’m complaining.
My experience has been that once you’re in the system and have got past the snarling gatekeepers, actual medical people are happy to treat and rebook you.
The (systemic) problem is that every person being treated or rebooked prevents someone else from being seen at all – your bumping to the top of the list knocked everybody else down a step, for example.
Yes I am aware of that, what am i supposed to do Surrender my space to someone more deserving?
Surrender my space to someone more deserving?
The only way to make the shit show work is to get hold of the contact details for everyone with whom you deal and don’t lose them. I book everything, for my daughter, direct and I make sure they send me a confirmation letter, as wasteful as it is. Once you get past the, hopeless, bureaucracy the NHS can actually work quite well.
I was able to make my unexpected follow up physio appointment right there and then with the physio receptionist .
.
I’ve now spent all morning trying to change it over the phone.

Main hospital switchboard starts off with the same old 3 minute covid/visiting restrictions recording before you even start the multiple choice by numbers attempting to get through to that same receptionist.
I remember years ago trying to phone the ward where my mother was in Glos Royal Hospital. They had a speech recognition (haha) switchboard and every time it asked which ward i wanted and i replied Nineteen, it came back with ‘You want the Canteen. Putting you through now’. It was impossible to get through to the ward!
Sounds like my own hospital is still using that same voice recognition technology.
They have direct numbers but it is a mother and father of a job trying to get hold of them. Keeps loads of pointless bureaucrats in a job though.
Yes, in some cases waiting lists are normal or short.
Needless to say, Facebook censors any link to this story…
The metaverse knows all!
Well, it’s only the BMJ. Not a prestigious journal at all.
Get Prince Harry on the case.
Apparently this Prince of Mispeak has been appointed a Misinformation Commissioner at the Aspen Institute (counts as a university it would seem).
Times, Mail, Independent and others.
So how was tested if someone of the participants of the study had Corona, with the extrem reliable PCR test ?
So from this we can infer that the jab increases the incidence of heart attack by 3x?
And, am I correct that all participants were under 50 and had no clinical vulnerabilities?
In this case, a 3-fold increase is really quite something.
Nothing can be trusted in this pandemic!
This is doing nothing for my vaccine hesitancy.
Does this explain why everyone is so determined to avoid having a large unvaccinated control group?
Yes, and also why there was a subsequent rush to inoculate the untouched control group in the pseudo-trials, to cover the tracks.
Another potential explanation could be that there never was a coof virus. If everyone has antibodies as a result of the jab, it’s (theoretically) not possible to say with certainty whether there were any naturally acquired antibodies in the mix. They just point to the jab antibodies and say they’re the same as natural ones. And the gravy train keeps on rolling.
Prof. Bhakdi noted a while back that in 99% of people the antibodies stimulated by the vaccines are of the type the body produces in response to an antigen it recognizes from a precious infection.
https://www.youtube.com/watch?v=bHoRCfP6urM
To be clear, this doesn’t necessarily mean they’ve already encountered the SARS-2 virus – it’s lik ly just cross-immunity from other coronaviruses.
The point, though, is that as long as your health is decent, then you’d produce these antibodies on encountering the SARS-2 virus, whether you’d received the vaccine or not.
Maybe there’s a case for stimulating this response via the vaccine for people in poor health, so they have antibodies ready should they encounter the real thing. But antibodies don’t last more than a few months. And if you’re one of the vast majority in decent health, it’s all moot anyway.
Yes, and quite a lot of us are being cajoled into using a potentially hazardous product for no real benefit, without any prior assessment as to whether we are already essentially immune in the first place. That is, either having dealt with the real thing, or the existence of enough cross-immunity from things like 229E, HKU-1 etc.
Al Jazeera y00tube is showing (on shorts) the CCTV clip of the Liverpool taxi bomb.
This blured and shaky clip has been seen all over the place yet y00tube chose to ‘age restrict’ Al Jazeeras upload saying it won’t play on ‘shorts’ but when the ‘play’ button is pressed . . It does. wtf?
Who knows. That ‘incident’ definitely ain’t as its been presented though.
The BBC broadcast some public sector cluts expressing his condolences to the ‘dead persons’ family.
He means the pretend Italian, pretend convert to Christianity who they say ‘may have had mental health issues’ on the basis that he had been Sectioned as a result of knife crime.
The ‘dead person’ who strapped a bomb to himself in an attempt to commit mass murder of Veterans at a Remembrance Day ceremony in a Cathedral.
The exploded detonator only damaged the Hero Taxi Drivers eardrum so the ‘dead person’ probably burned to death in the locked cab. My heart lies bleeding.
I can think of at least 5 people I’d like to add to this statistic, actually sod it, how many MPs are there? Let’s hope it’s delayed treatment and not poisoning.
https://www.telegraph.co.uk/news/2021/11/16/nhs-delays-height-pandemic-linked-thousands-extra-non-covid/
I couldn’t write everything in my BMJ’s RR; many adverse reactions were not reported (some people recruited in the trial provided evidence of that), the centre based in Argentina didn’t report anything, while they faced some serious adverse events (including life-threatening cardiac events), Imagine if they could hide something as expected as an allergic reaction (which is quite standard for a product being injected), how many other horrible reactions have been put under the carpet ?
Surya
Thank you for your bravery in challenging the status quo. I am in the process of trying to get official exemption from the “vaccines” for my family and your work has given me greater confidence to fight.
In any normal world, not befuddled and deluded by greed, propaganda, arse-saving and Prime and other Ministerial amour propre, the barest sniff of the Yellow Card, VAERS, European and other AR notification systems, plus the other stats and research now emerging since this stuff first came to be a curse on the human race, the sirens would have sounded, the red flags hoisted and the jollop outlawed, for its harm and lethality.
I hope that humanity can wrest itself from its torpor and stupor, such that “he who sows the wind, reaps the whirlwind” and condign punishments and restitutions are applied.
So even the Hopeless are now full of hope
“Hope springs eternal in the Human Breast”.
Rather less inspiring is the Vodafone ad on TalkRadio, encouraging people to hand in old iOS/Android phones (“less than five years old”) so that people (rough quote) “can be be brought out of the digital wilderness” with these phones and free SIMs and time. I suppose this particular corporate puppet has had a Government hand up its backside so that more people, especially older ones, have a Devil’s Invention upon which to show their Vaccine Passport/Status.
Learn to root your android phone. Will be needed to host your own node in a VPN.
I did, with my old Xiaomi, which is now in rather more than its component parts. I had to root it anyway to clean out the dangerous and unwanted stuff. Now I have a Nokia dumbphone, which connects only to the phone network, and spends almost all its time in a Faraday Cage aka metal box.
Yes I saw that and it made me want to vomit.
We need people to be spending less time looking at screens, not more.
It is the people who are lost in social media who are “in the wilderness”.
As someone who works in IT it pains me to see IT companies using technology to deliver “solutions” to non-existent problems. It gives IT a bad name.
Do you mean they intend to gift these handed in phones to the phoneless?
What happened to all the handed in laptops that were supposed to likewise be gifted to underprivileged kids schooling from home 2020 summer term?
Well I trusted those that say the trial was fraudulent
Pfizer are one of the biggest organised crime syndicates in the world with a track record of wholesale fraud, routine bribery and systematic corruption resulting in record breaking fines for criminal convictions together with huge civil payouts. But this is not unusual. In fact, this is how the Rockefeller initiated, Big Pharma organised crime racket has, over the last decade or so, accrued so much leverage over politicians. A nexus of power which culminated in the 2020 globalist coup where objective observers witnessed a coming together of Big Pharma, Big Tech, their political puppets and media outliers on a scale never before seen – and now we are living with the consequences.
Thank you. Excellent, spot-on, succinct post. I’m copying this to my “Covid file” of great quotes.
Thanks for that but its just stating the obvious really.
Pfizer is the only vaccine left standing – AZ is basically finished in most of the developed world at least; J&J is not approved in Europe; Moderna is in the process of being banned completely or for young people in many countries.
To date Pfizer has managed to avoid the level of scrutiny applied to the other vaccines but I don’t think that is going to last for much longer. The BMJ paper and Peter Doshi speaking out against the COVID orthodoxy was a turning point.
I do hope you’re right on that last point.
I told a not particularly bright friend about 100% vaxxed Gibraltar cancelling Xmas.
After that had sunk in he responded by saying
“Suppose that means we’ll have to get some new ones then”.
Fortunately for him our mutual GP Practice seems only have used AZ for initial jabs so he can be in line for Pfizers effort as a booster.
Janssen jab received its conditional authorisation throughout the EU on 11 March 2021.
Facebook still censoring any mention of the BMJ article.
I appreciate the work, but at this point we’re doing sartorial analysis of the tread on the boot that’s stamping on our faces.
Shock.
A major bigpharma unblinds a trial.
They do this every bloody time.
Completely prevents any undesirable inferences to be drawn you see.
Oh and while we’re at it, bigpharma is never bothered in the slightest by having to pay fines of any description.
The only thing that scares them is “Discovery and Inspection” of their commercially sensitive documentation – which can only arise in litigation – hence the present immunity from litigation re mrna “vaccine”
Takes years mind,- fines/lawsuits – take a bow :-
PFIZER Bextra 2.3 Billion dollars.
MERCK Vioxx 4.85 Billion dollars.
(Not to mention 50,000 + deaths)
JOHNSON & JOHNSON Risperdal 2.0 Billion dollars.
GSK Pandemix (Lol) TBA- continuing…..
The above are just token samples.
Can anyone tell me why so many trust them!
They cannot have exclusion from criminal prosecution. It requires the will of real prosecutors and real courts to delve into this, but disclosure could be compelled, there is enough evidence at this point for courts to be able to force pfisser (and the rest) to disclose all the information they have. There is zero doubt in my mind they have information which they have deemed “irrelevant” to the trial (i.e. withheld).
The same applies to the famous contracts with governments. Courts can declare contracts void as against the public interest and can void them ab initio, meaning any arbitration clauses are also void. Pfisser could then be required to appear in the courts of country x to pursue breach of contract should it so wish. Such country would then, in the meantime, be entirely free to set requirements as to who must appear in person (for example, top executives) and also lay a few criminal charges for the executives to answer should they set foot in the country.
There are legal options, it takes a will. I guess it will take a few more months and a lot of deaths and injuries from the vaxx, including among friends and family members of the legal powers, for them to be willing to take action.
Corporate Manslaughter gets senior managers/executives sent to gaol I believe.
All true, but it takes ‘class actions’ usually in the US and its decades before an answer. There is so much money involved the pharmas spend $bns on lawyers ( and inducements) just to slow everything to a snails pace.
Johnson & Johnson, plus the class action over their carcinogenic talcum powder.
This from Amazing Polly about the clinical trials: vaccine injuries cover up. It’s pretty mindblowing. https://www.bitchute.com/video/Szi5AcqpqEhz/
so more died on the vaccine arm than the placebo arm? and this shows the vaccine works?
So… 22,085 were selected for the vaccine, 22,080 were selected for the placebo. That’s odd… why the 5 people difference? Why not 22,083 and 22,082? But then, after a number of people left, 22,080 were given the first dose, and 22,080 were given the placebo. Well… isn’t that one big coinkydink. Just enough people happened to drop out of both arms that both cohorts had the exact same number! What are the odds of that? 26 withdrew from both. 5 people in total had adverse reactions to merely being selected. How can you look at that and not think there’s something weird going on? Did no one ask any questions about the numbers before approval? Probably not.
There is an ivermectin panic on the big tech and MSM right now. Massive articles from MSM on Ivermectin trying to push a danger narrative and also negative press on Americans Frontline Dr’s, again, to keep the Covid narrative alive. Just go to the Goog and type ivermectin then look at all the panic news articles. We are over the target. Big-Pharma is panicking. This medicine has been widely used by humans without any problems for 40 years. It’s inventor won a Nobel Prize after 20 years of successful use and after 100 million people were cured of a broad spectrum of problems without any side effects. Get your Ivermectin while you still can! https://ivmpharmacy.com
To prevent one COVID-19 related death, Pfizer admits their vaccine KILLS at least three people by causing cardiac arrest
Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine
https://www.nejm.org/doi/suppl/10.1056/NEJMoa2110345/suppl_file/nejmoa2110345_appendix.pdf
See Table S4 below.

Common sense dictates that the harms of this vaccine far outweigh the benefits.
The probability of this outcome of 4 cardiac arrests in the vaccine group versus vs. 1 in the placebo group being a chance occurrence, is only 1.5%.
1/22000 in the placebo group is used as background cardiac arrest death rate.
(((21999÷22000)^21996)×((1÷22000)^4)×(22000!))÷(21996!×4!) = 0.0153 or 1.5%.
So we have 98.5% probability that the 3 excess cardiac arrest deaths were CAUSED by the vaccine.
Since regular calculators cannot perform this calculation, you can use WolframAlpha to verify.
https://www.wolframalpha.com/input/?i=%28%28%2821999%C3%B722000%29%5E21996%29%C3%97%28%281%C3%B722000%29%5E4%29%C3%97%2822000%21%29%29%C3%B7%2821996%21%C3%974%21%29+
Yes everything – the RRR (Fake%) numbers are 95%, but the ARR (True
%) numbers for Pfizer are 0.84% – less than 1% efficiency – if you are going to bet your life on Covid having a 99.92% chance of killing you and the Pfizer jab of 0.84% in saving you, then you are certainly someone I would bet my money against, to “win” all of yours – your horse becomes my horse, just like that!!
We really need to have an alternative plan in place to deal with the next Coronavirus outbreak, one that is 100% effective, costs nothing in materials(all on hand) and is simple to do and also is a really quick way to find out if we have just got a new viral strain, or not anf kill it dead quick:
I picked up a dose of a viral infection the day before yesterday, it might have been when I was out shopping or at the doctor’s surgery – I have NOT had the vaccine and won’t if I can avoid it “here”
I had the peppery feeling in my nose, which usually suggests a cold, so I did my salt water sniffle, which I repeat below, and had a slight reaction to the salt, so I did my salt water treatment again, straight afterwards and this time I had a strong reaction to the salt, from my left nostril right up into my hair line – I would describe it best if I could have split my head in two equal halves, the left half was the sore side, in my brain and to the top of my skull the right half was clear – I have NEVER had that experience previously with my salt water sniffle.
I left the salt in my head for another couple of hours and I had a slight head-achy feel at the top part of my head, which another salt water cure erased and afterwards I blew out my nose and flushed away, washing my hands afterwards.
This morning I had my salt water sniffle and no reaction at all to it, which means I had cleared the viral infection and just to be sure I had another salt water sniffle a couple of hours ago and still OK, so I have passed the virus – killed it dead in my head and of course I won’t get Covid anything – bearing in mind that the virus could have been for any viral illness, Alzheimers, Chicken Pox, Legionnaires Disease, The Flu or Coronavirus relating to Covid or just about anything – have to see if any “new” viral infection is reported locally over the next few days or so.
My point being that if you think you have even the slightest reason to think you have picked up a viral infection, do my free salt water cure and stay healthy and safe, because this works and why I never get sick from viruses these past 27 years and I never have vaccines for Flu or Coronavirus type illnesses – because I remain well and am never ill, so what is the point?
The assumption is that the only way to deal with Covid, is after it arrives as Covid in your body, after the initial cold infection, in your head, some 20 days earlier
.Why is that?
Kill the Flu or Coronavirus in the head, soon after getting the virus in the nasal passages inside the head, the brain bulb and brain stem, etc, with my free salt water cure, which flushes out the nasal passages (so no Long Covid) and kills off the Coronavirus infection, immediately, or during the 10 to 14 days of self isolation.
No infection in the head, no Covid – it is as simple as that.
Then the purpose and functions of the vaccines, ceases to be a problem and you simply can’t get sick and won’t ever get Covid.
Mix one heaped teaspoon of “iodine” table or sea salt in a mug of warm or cold “clean” water, cup a hand and pour some of the solution in, then sniff or snort that mugful up into your nose, spitting out everything which comes down into your mouth, from the back of your throat, by so doing, you flush out your nasal cavity, where Coronavirus lives.
If you get a burning sensation (which lasts for 2-3 minutes) then you have a Coronavirus infection.
When the soreness goes away, blow out your head with toilet paper and flush away, washing your hands afterwards and continue doing my salt clean water nasal cavity flush cure, morning, noon and night, or more often, if you want, until, when you do my free salt water cure, you don’t experience any soreness at all in your nasal cavity inside your head.
While you are at it, swallow a couple of mouthfuls and if you get a burning sensation in your chest, then you are killing the Covid/Pneumonia there too, so keep it up, each time you do a salt water sniffle, until the soreness in your head and lungs goes away – job done.
When you flush your head with the salt water remedy, it should feel like you are flushing your head with water – no reaction felt at all.
I have been doing this for 27 years and I am never ill from viruses and there is no reason for anyone else to be either and of course, I never have vaccines – what is the point?
You don’t need to be tested to see if you have a head infection, you will know instantly if you have or not, with my free salt water cure
Keep safe and stay well with my free salt water cure
Richard
This article by a German Physician sets out how mRNA vaccines work and explains just why the Adverse Reactions to it have been as bad as they have. It suggests that they are set to get much worse with ‘booster’ jabs.
https://notrickszone.com/2021/11/17/doomsday-vaccine-german-physician-corona-vaccines-a-programmed-self-destruction-of-the-body/
A comparison between industrial products and the medical trade.
As a retired engineer who spent a lot of time working on systems that were intended to achieve high-end “Safety Integrity Level” values, it seems to me that the claims made by the Pharmaceutical trade re the novel product are simplistic to say the least. If anyone is interested, search for the term quoted, and relevant standards like IEC 61508.
It may well be true that there is a significantly different attitude to the meaning of the concept of “safety” in medicine compared with product engineering – after all, if the choice is to take a chance, or else you’re dead, the sums are a bit different. Maybe that is part of the issue.
However, it often looks as if some aspects of medicine live on a foreign land compared with much of industry. Of course they do use tools & instruments that have to meet the relevant standards, but when it comes to advertising that a certain drug is considered to be “safe and effective”, is that reasonable behaviour, compared with what the manufacturer of something else has to do? Indeed, does it actually meet normal advertising standards, given the published results? A cynic might ask: how bad do things have to be for ‘unintended side effects’ to morph into manslaughter?
It’s like: would anyone trust a Convicted Paedophile to babysit!