I have previously written about a tendency by medical study authors to downplay their results if they don’t conform with the official narrative regarding the COVID-19 vaccines. A study done in Iceland and published last summer found that double-vaccinated individuals were 42% more likely to become reinfected than others. But in their conclusions the authors called this just a “slightly higher” probability.
Now, a new study is out, published in the BMJ, that deals with female menstruation problems following vaccination. Nothing to worry about, according to mainstream media reporting. Indeed, in their conclusions the authors say:
Weak and inconsistent associations were observed between SARS-CoV-2 vaccination and healthcare contacts for bleeding in women who are postmenopausal, and even less evidence was recorded of an association for menstrual disturbance or bleeding in women who were premenopausal. These findings do not provide substantial support for a causal association between SARS-CoV-2 vaccination and healthcare contacts related to menstrual or bleeding disorders.
No reason to worry – really? Let’s take a look at the results section now:
2,580,007 (87.6%) of 2,946,448 women received at least one SARS-CoV-2 vaccination and 1,652,472 (64.0%) 2,580,007 of vaccinated women received three doses before the end of follow-up. The highest risks for bleeding in women who were postmenopausal were observed after the third dose, in the 1-7 days risk window (hazard ratio 1.28 (95% confidence interval 1.01 to 1.62)) and in the 8-90 days risk window (1.25 (1.04 to 1.50)). The impact of adjustment for covariates was modest. Risk of postmenopausal bleeding suggested a 23-33% increased risk after 8-90 days with BNT162b2 [Pfizer] and mRNA-1273 [Moderna] after the third dose, but the association with ChAdOx1 nCoV-19 [AstraZeneca] was less clear. For menstrual disturbance or bleeding in women who were premenopausal, adjustment for covariates almost completely removed the weak associations noted in the crude analyses.
So, actually significant risk for postmenopausal even after adjustments, but for premenopausal the “weak associations” were removed after adjustment for covariates. Why those huge adjustments? Before adjustment they found statistically significant increases of up to 44% – but that top figure was ‘adjusted’ away to just 4% (see Table 3). Yet even after these heroic adjustments there was still a 25% increase in menstrual disturbance following the first dose.
Anyhow, let’s look at the actual numbers by product for postmenopausal.
First Pfizer: adjusted risk (right-hand column) is 1.41 or 41% higher than the unvaccinated after 1-7 days from the third dose and 1.23 or 23% higher after 8-90 days. Both are statistically significant. “Weak and inconsistent?” Really?
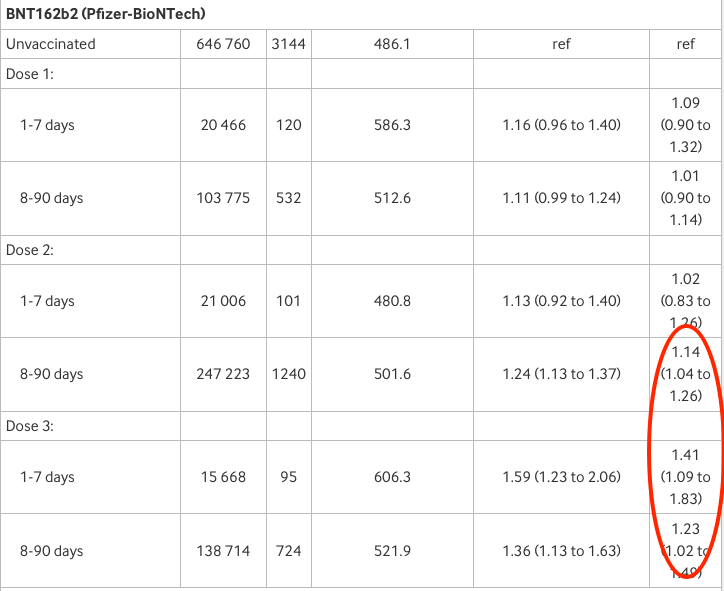
Now for Moderna: adjusted risk is 1.33 or 33% higher than unvaccinated after 1-7 days from first dose and also 8-90 days after the third (the latter is statistically significant). Again, “weak and inconsistent”?
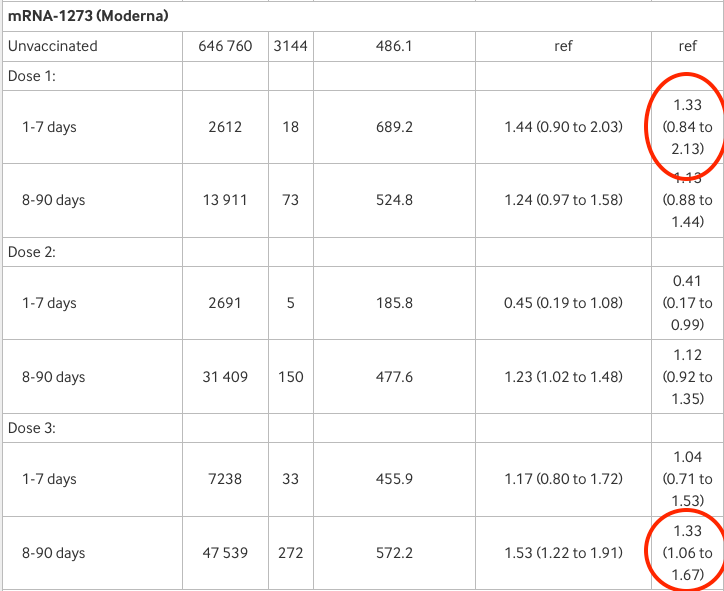
Finally AstraZeneca: adjusted risk is 1.24 or 24% higher than the unvaccinated 1-7 days after the first dose and 1.21 or 21% higher than unvaccinated after the second (though neither result is statistically significant).
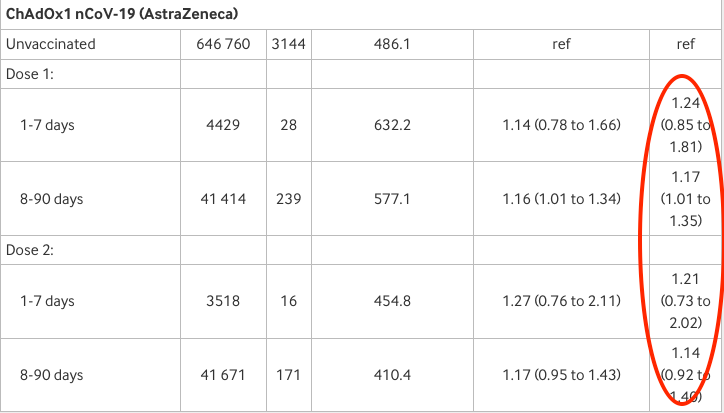
Last October, the European Medicines Agency finally recommended adding menstrual problems to the already long list of COVID-19 vaccine side-effects. It was about time, after the flood of reports from women. The results of the new study reinforce those concerns, as shown above.
The question that remains is why the glaring discrepancy between the actual results and the authors’ conclusions?
The authors know full well that most journalists neither read nor understand scientific studies; they know how their highest ideal of verification is appeal to authority (‘the authors say, therefore it is true’). Every scientist knows this. Therefore, it is the authors’ responsibility to correctly portray and highlight their actual findings. But instead they try to hide them.
Why?
Is the answer to be found in the ‘competing interests’ section, perhaps?
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: MG reports personal fees from AstraZeneca, Gilead, GSK/ViiV, MSD, Biogen, Novocure, Amgen, Novo Nordisk, outside the submitted work. SL reports consulting for Scandinavian Biopharma and is an employee of AstraZeneca since 16 January 2023. The work in this article was performed before this employment commenced. FN reports prior employment at AstraZeneca until 2019, and ownership of some AstraZeneca shares. MB and YX declare no competing interests. AS reported participating in research funded by governmental agencies, universities, Astellas Pharma, Janssen Biotech, AstraZeneca, Pfizer, Roche, (then) Abbott Laboratories, (then) Schering-Plough, UCB Nordic, and Sobi, with all funds paid to Karolinska Institutet, outside of the submitted work. RL reported receiving grants from Sanofi Aventis paid to his institution outside the submitted work; and receiving personal fees from Pfizer outside of the submitted work.
If there are still any real journalists out there, how about getting in touch with the authors and actually asking? Would be a nice change, wouldn’t it.
This article was first published on Thorsteinn Siglaugsson’s Substack newsletter, From Symptoms to Causes. You can subscribe here.












To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
Thanks, Thorsteinn.
But I’m afraid you’d need to hold the hand of every single “journalist” through these numbers.
I suspect 99% can’t add up their daily shop.
what! inconvenient reality being silenced!!
preview well worth a watch….
https://www.followthesilenced.com
Way before Covid, I had learnt to read journal articles starting with the “competing interests” section, then methods, then results and conclusions last.
Covid certainly reinforced my policy.
It also means that the vast majority of research can be happily consigned to the dustbin.
I never cease to be amazed when the “conclusions” of a research paper are diametrically opposed to the “results”, purely in order to keep the financial masters happy, and score a publication.
I persuaded my 62 year old wife to complete a Yellow Card incident when she suffered from it.
I unfortunately couldn’t previously persuade her not to have the jab as she had a holiday of a life time booked in the Maldives with her friends and that is the only reason she had it.
So what was the nature of the adjustment for covariates? This is the key question, but Thorsteinn doesn’t say. Were they explained in the paper or not?
If you automatically assume you’re being lied to, you won’t go far wrong.
“If you automatically assume you’re being lied to, you won’t go far wrong.”
My view entirely. Always assume that announcements from official bodies, whoever they are, are a pack of lies until said authorities prove differently. As a default position it is highly recommended
22.1% excess deaths here in the UK in week ending 21 April..
2540 non Covid excees deaths in 1 week.. More excess deaths than at the height of the so called “pandemic”
Where’s the debate?, wheres Whitty et al with their graphs and daily broadcast now???
The only politician who raised these concerns gets thrown out of Parliament, even though he was democratically elected by his constituents.
Similar excess deaths in all vaxxed countries
A highly effective “vaccine”??? No it wasn’t.
Cancer relapses through the roof………… Bio weapon would be a more appropriate term.