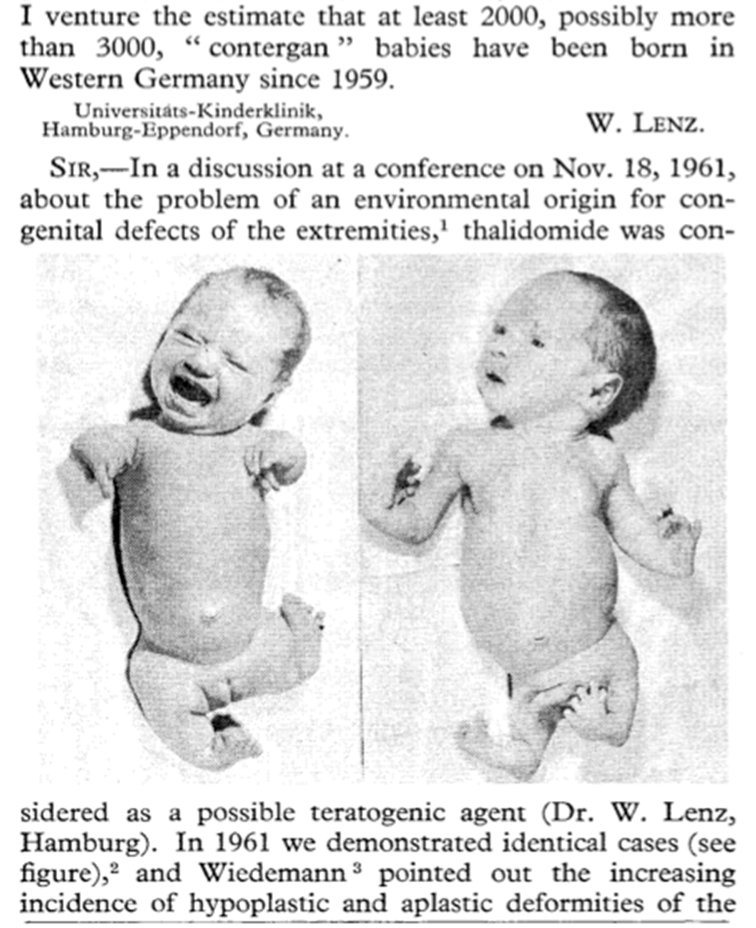
Any student of pharmacy should be familiar with the thalidomide tragedy and most of the public are at least vaguely aware of this disaster. Thalidomide was a sedative that was found to be useful in preventing morning sickness in pregnant women. It was developed by the German company Chemie Grunenthal in 1954 and was introduced onto the market in the U.K. in 1958. However, doctors started to notice an increase in the number of babies being born with birth defects and, in December 1961, the Australian gynaecologist, Dr. William McBride published a short letter in the medical journal the Lancet highlighting his concerns that thalidomide might be responsible for these birth defects. At the same time, the German doctor, Dr. Widukind Lenz noted similar concerns and, in November 1961, warned Chemie Gruenenthal of his fears. The drug was withdrawn from the market at the end of 1961 but disputes over the question of whether thalidomide did or did not cause malformations went on for months. Chemie Gruenenthal continued to deny the teratogenic effects of thalidomide for years, but there was suspicion that this was not due to honest ignorance but in order to weaken the accusations against the company (see this extract from a lecture given by Dr. Lenz for details).
Why is this relevant to the withdrawal of the AstraZeneca Covid Vaccine? Well, as any good pharmacy student will also know, the thalidomide tragedy led to the formation of the Committee on Safety of Drugs (1963) which was set up by Sir Derrick Dunlop. This became the Committee on Safety of Medicines (1968) then the Medicines Control Agency (1989) and finally the Medicines and Healthcare products Regulatory Agency (MHRA) in 2003. The MHRA is the U.K. agency that monitors the safety of drugs and approves them for use on the U.K. market. It also monitors safety of medicines available to the public through the Yellow Card Scheme.
Prior to the set-up of the Commission on Safety of Drugs, companies could make drugs and sell them to the public without undertaking testing and without the drug formally being approved for use in patients. In 1968, with the introduction of the Medicines Act, medicines needed to be licensed before being introduced onto the U.K. market. Companies needed to show that they were safe for use in patients through proper testing (pre-clinical testing and clinical trials), quality testing and pharmacovigilance (post-marketing safety monitoring). The AstraZeneca Covid Vaccine, Vaxzevria, went through an accelerated version of this process and was deemed safe for use in patients on an emergency basis, with the first patient injected in January 2021.
Problems with any drug should be picked up through the Yellow Card reporting scheme. This scheme was introduced into the U.K. in 1964 in response to the thalidomide tragedy. The Yellow Card scheme allows healthcare professionals and the public to report suspected side-effects or adverse reactions associated with medicines, vaccines, herbal remedies and medical devices. The information gathered through the Yellow Card Scheme helps regulatory authorities, such as the MHRA to monitor the safety of medicines and to take appropriate regulatory actions (e.g. withdrawal of the medicine) to protect the public.
For vaccines, the number of reported side-effects tends to be fairly low (in the hundreds) and the number of deaths reported tends to be in single figures. These vaccines tend to mainly be given to babies and children as part of the standard vaccination protocol. The estimated reporting rate of side effects per 100,000 doses is around 10 for such vaccines (for example, see the 2009 report from MHRA).
With the outbreak of Swine Flu, a mass vaccination programme was introduced in the USA in 1976, but with severe consequences. Approximately 1 in 100,000 vaccinated individuals developed Guillain-Barré syndrome according to CDC figures, and several deaths were also reported; the vaccine was withdrawn from the market when these concerns became too obvious to ignore.
There are lessons we should have learnt from this incident and, as Richard Fisher pointed out in his BBC article in September 2020, we really should have looked at this past mass vaccination programme and been forewarned. If you rush out a vaccine for a mass vaccination programme, as a Government you are under a lot of pressure to fulfil your promise of vaccinating the population and people dropping dead is really quite inconvenient.
In the U.K., approximately 50 million doses of the AstraZeneca Covid vaccine were given to approximately 25 million people. The vaccine was mainly used between January 2021 and July 2021, with the Pfizer vaccine becoming the primary vaccine of choice from the summer of 2021. Up until the end of September 2022, the MHRA Yellow Card Scheme received 246,393 reports of adverse reactions to AstraZeneca vaccine, with a total of 873,051 reactions and a massive 1,314 suspected fatalities related to the vaccine (the data were nicely summarised on the U.K. column website). Approximately 1% of patients who received the vaccine reported side-effects and five suspected patient deaths were reported per 100,000 patients injected.
If we compare that to the Swine Flu fiasco, where the vaccine was withdrawn when 1 in 100,000 patients developed the serious side-effect of Guillain Barre syndrome, it might reasonably have been expected that, with five suspected deaths per 100,000 patients injected, either MHRA or AstraZeneca might have considered withdrawing the product.
Should AstraZeneca vaccine have been withdrawn sooner? The company voluntarily withdrew the product from the market this week (May 7th 2024). It cites commercial reasons for withdrawing the product – which is fair since it has hardly been used in earnest in the U.K. since the summer of 2021 – and the fact that it is probably not effective against any current strain of COVID-19, also fair. However, this still raises the question as to why, with unprecedented safety concerns relating to this medicine, was it not withdrawn earlier? The Yellow Card Scheme highlighted these concerns. I fear that Sir Derrick Dunlop would have been very disappointed that we still haven’t fully learnt the lessons from thalidomide. Like Chemie Gruenenthal, will AstraZeneca continue to deny the side-effects of its vaccine, or will it continue to plead ignorance in order to weaken the accusations against the company?

Dr. Maggie Cooper is a pharmacist and research scientist.













To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.