I wrote here about the fact that MHRA’s definition of ‘safe’ is relative – “the benefits outweigh the risks” – whereas all other safety sectors define ‘safe’ in absolute terms – one in a million probability of death, one in a thousand probability of serious injury, etc. Other sectors define these up front and then investigate all safety incidents and take appropriate action to limit, modify or suspend the product if those absolute safety risks are breached in practice.
MHRA would doubtless argue that manufacturers have to put absolute safety risks in the Patient Information Leaflets which you find in the box. That’s true, there are probabilities (‘common’, ‘rare’, ‘very rare’, or one in 100, one in 1,000 etc.) However, there’s a big problem: those aren’t thresholds which, if breached, trigger MHRA to take action to limit, modify or withdraw the medicine. Instead, MHRA just seems to list all the emerging side-effects in the Patient Information Leaflets and carries on.
To illustrate this, I’ve had a poke around the evolving versions of the Patient Information Leaflets for the Covid vaccines since rollout started. It’s terrifying. I’ll show you them in a moment, but what it’s been doing as Yellow Card reports rapidly accumulated is to:
1. Move already known adverse events between frequency categories if they turn out to be more common than they thought based on the (much shorter than normal) clinical trials.
2. Add new adverse events which only emerged during rollout (i.e., they weren’t identified in the clinical trials).
Worse still, months after rollout started, MHRA:
3. Added a new category (‘1 in 10,000’).
4. Added some words on the risk of death (which wasn’t previously mentioned at all).
You can see this in all its glory by using the Wayback Machine for Pfizer/BioNTech, AstraZeneca and Moderna. Go to the version of that webpage archived on the various dates shown and then find the corresponding Patient Information Leaflet.
Space doesn’t permit showing you them all, but just spot the differences between the first AstraZeneca PIL (December 30th 2020) and the most recent (November 2023).



I mentioned that the original PILs didn’t include the risk of death. This was only added later to the AstraZeneca PIL (on April 18th 2021) and the Moderna PIL (on September 15th 2023). But it wasn’t actually quantified: it was just introduced as “fatal cases have been seen” or “some cases had a fatal outcome”. It is still not mentioned in the Pfizer PIL despite at least one coroner citing that vaccine as the cause of death. This is the antithesis of informed consent.
I found something else. The Human Medicines Regulations state that: “The licensing authority may establish a list of medicinal products that are subject to additional monitoring” and “the summary of product characteristics and the package leaflet must include a symbol and statement as follows: “▼ This medicinal product is subject to additional monitoring.” Using the Wayback Machine, I found that the COVID-19 vaccines were not designated as such until months after Temporary Authorisation: Moderna on April 1st 2021, AstraZeneca on July 7th 2021 and Pfizer on July 14th 2021. It is not clear why the COVID-19 vaccines were not designated as worthy of additional monitoring from the start, or, if they were, why the Black Triangle was not included in the Patient Information Leaflet (which would be seem to be a breach of the Human Medicines Regulations).
Finally, let’s go back to MHRA’s relative definition of safety: “benefits outweigh the risks”. That might even have been fine if the benefits from the Covid vaccines got better as the risks got worse but, as we all know, the benefits got worse, too. Effectiveness turned out to be less than the manufacturers originally claimed and it waned within months – hence booster after booster. And the vaccine effect on transmission turned out to be illusory. But MHRA doesn’t want you know about the benefit side of the equation. For example, here’s a slide from an internal MHRA presentation just released under an FOI:
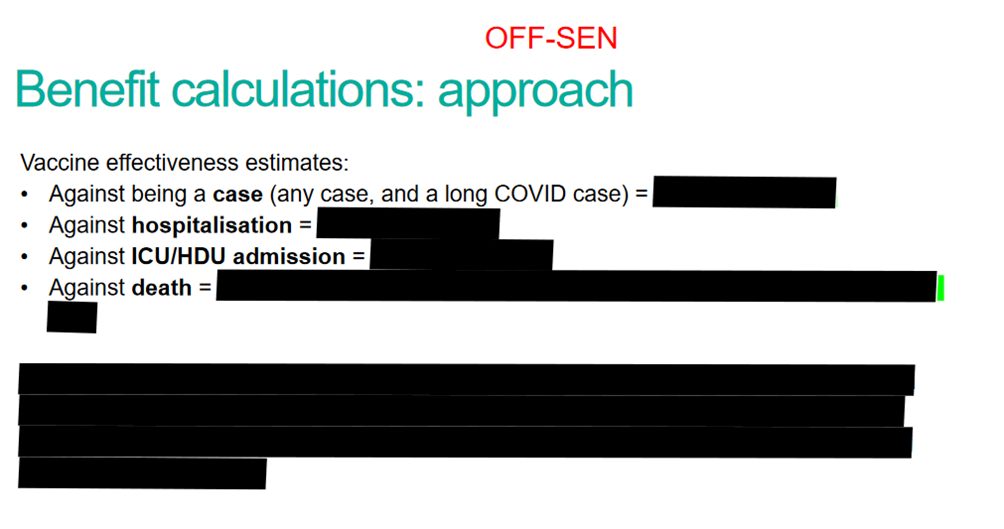
I’ve got more like this. Redaction after redaction. MHRA claims it’s hiding the data to protect commercial confidentiality. A suspicious person might think it’s got more to do with the ongoing legal cases against AstraZeneca.
It’s outrageous. MHRA is supposed to be protecting us from pharmaceutical manufacturers, not the other way round.
Until Nick retired a few years ago, he was a Senior Civil Servant in the Ministry of Defence responsible for the safety and effectiveness of ammunition used by the Armed Forces. He is co-author of the Perseus Group report on U.K. medicines regulator the MHRA.










To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.