One of the most critical concerns in medicine is the massive influence of the pharmaceutical industry over the medical profession. Our antivirals series has shown this pervasive action.
Bias exists when professional judgements from registered medical practitioners concerning a primary interest, such as the welfare of patients, may be influenced by secondary interests, such as financial gain or loss. Conflicts of interest can affect how healthcare professionals use, see and interpret evidence. Conflicts of interest, perceived or real, can also cause a huge loss of public trust in medicine, healthcare and government.
When it became clear that TV doctors Ranj Singh, Nighat Arif and Philippa Kaye had all received thousands of pounds in contracted service fees from AstraZeneca, there was, unsurprisingly, an outpouring of concern. All three doctors had been vocal in their public support for COVID-19 vaccines, of which AstraZeneca’s Vaxzevria was one of the first to become available and widely used in the U.K.
All three have insisted, via X, that their payments were for promotional work relating to AstraZeneca’s nasal flu vaccine in winter 2021 only and not their COVID-19 vaccine. The Human Medicines Regulations state that celebrity endorsement of drugs is not allowed, so it is unclear how this campaign stayed on the right side of the legislation.
Whether an individual doctor’s paid promotion of a vaccine for a respiratory disease should be considered a conflict of interest when publicly recommending other vaccines for respiratory disease is probably a debate for another day.
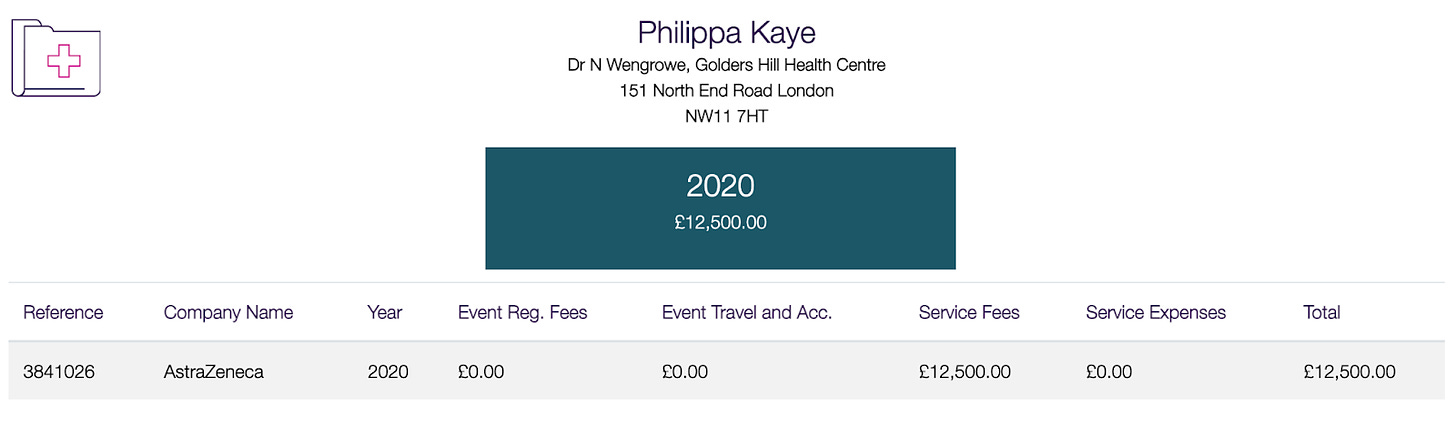
According to the Association of the British Pharmaceutical Industry (ABPI)’s Disclosures U.K. database, AstraZeneca’s payment of £12,500 to Dr. Philippa Kaye was declared in 2020. Since then, Dr. Kaye has publicly supported AstraZeneca’s COVID-19 vaccine and downplayed side-effects generally from all COVID-19 vaccines, saying the side-effects “are a good thing” and “your body showing you that its immune system is working”.
Also listed on the ABPI’s Disclosure U.K. Database is Dr Ranj Singh’s payment of £22,500 by AstraZeneca, declared in 2022. He appeared on BBC Morning Live on May 7th 2024 to discuss legal action over injuries and deaths linked to AstraZeneca’s vaccine. In both cases, as the doctors failed to declare their previous paid work with AstraZeneca, we assume they must be in breach of their contracts due to Clause 24 of the ABPI’s Code of Practice, which deals with Contracted Services. It states that all contracts for such services must include a requirement that the contractor declares his involvement with the contracting pharmaceutical company whenever he speaks publicly about any matters related to that pharmaceutical company.
If the declaration of interest was not included in AstraZeneca’s contract with the TV doctors, then the pharmaceutical company would be in breach of the ABPI Code and must self-report these incidents to the Prescription Medicines Code of Practice Authority (PMCPA) as breaches of the code.
Concerns about payments from pharmaceutical companies are not confined to these three TV doctors. The ABPI Disclosures database also reveals that AstraZeneca declared a payment of £5,892 in 2022 to Jonathan Van-Tam, the former Deputy Chief Medical Officer for England and member of the U.K.’s Vaccine Task Force – a payment which Van-Tam has not declared as a potential conflict of interest in academic work such as this SARS-CoV-2 study. This is not the only time that concerns have been raised over Van-Tam’s close connections to industry, with his appointment in 2023 by Moderna as a consultant causing controversy around the so-called ‘revolving door’ between public and private sector jobs.
In 2017, Tom warned: ‘The U.K. turns to Witty, Vallance, and Van Tam for leadership. Revolving doors‘? The consequence was predictable: the solution to every illness is more pharmaceuticals.
Meanwhile, another U.K. Government adviser and frequent media commentator on the benefits of COVID-19 vaccinations, including for children, Professor Peter Openshaw, was paid £2,251by Pfizer and £6,012.78 by Moderna in 2022. On September 14th 2021, Openshaw stated on BBC Today that, in relation to younger age groups having the vaccine, “If you want myocarditis, get Covid. Although there is a signal from vaccination, the signal from catching the virus is much greater in terms of inflammation of the heart.” At the time, a pre-print study put the risk of 12-15-year-old healthy boys experiencing cardiac adverse events such as myocarditis after vaccination at around four times higher than after being admitted to hospital for COVID-19.
It’s also worth noting that an individual practitioner’s obligation to declare involvement with a pharmaceutical company starts when the contract is signed, not when actual payment is made. Furthermore, the exact payment date to individual practitioners is not currently disclosed. Disclosure to the ABPI needs only to be made within six months of the end of the year in which payment was made. Meaning actual payment could be up to 18 months after the actual payment and even longer after the contract was signed. Therefore, we need much greater clarity about the exact timing of payments to highly influential doctors and the timings of when the negotiations started.
Arbitrary time limits severely hamper research into payments made by the pharmaceutical industry for disclosure imposed by the ABPI. Currently, its code of practice only requires data to be published for three years, after which they are deleted. As pointed out in a comprehensive article about the ABPI’s Disclosure U.K. database, written by retired pharmaceutical physician Dr. Alan Black, the life cycle of a medication, from preclinical phases to active marketing, can run into decades, so “time-limited transparency is not genuine transparency at all”.
Healthcare professionals are also free to refuse consent for a payment to be attributed to them in the Disclosure U.K. Database. In addition, any payments made to journalists are not required to be attributed to specific individuals; instead, they are included in an aggregated amount paid to all journalists in a given year.
Serious reform is needed and has been for a long time. Not much seems to have changed even since the ‘First Do No Harm: Independent Medicines and Medical Devices Safety Review of 2020‘, which declared the need for “a statutory requirement similar to the Physician Payments Sunshine Act 2010 in the U.S.” to properly deal with the thorny issue of “transparency of interests”.
As two registered medical practitioners, we urge Parliament to pass urgent legislation considering non-disclosure of any relationship between medical practitioners, media, pharmaceutical industry and Government a crime.
As the consequences of what has been going on for decades become more evident through the magnifying glass of the Covid panic, there is an urgent and vital requirement to salvage what is left of the credibility of medical practitioners.
Dr. Carl Heneghan is the Oxford Professor of Evidence Based Medicine and Dr. Tom Jefferson is an epidemiologist based in Rome who works with Professor Heneghan on the Cochrane Collaboration. This article was first published on their Substack, Trust The Evidence, which you can subscribe to here.












To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
A little bit of good news helping to clip Mr G’s wings a bit. Let’s hope this is just a start.
And surprising, given that Gates and the Tronald met for 3 hours. I assumed they were planning scamdemic 2.0 or the idiocy that mRNA will cure cancer (cancer is toxins destroying your liver and organs).
Still waiting for RFK to get off his ass and make mRNA stabs illegal, as well as shuttering the ‘normal quackcine’ programs. Autism, child cancers, learning disorders and all that.
Never mind any other issues, the further from Gstes the better.
Speaking of the cult of vaccines, the new Joe Rogan podcast with Dr. Suzanne Humphries is eye opening… Highly recommended!
Clean water and DDT will do more for humanity than Gates ever will.
Step Two:
Reading this in 2016 was when I realised Gates is either a crook or a thicko or both:
https://www.gatesnotes.com/smells-of-success
And what is interesting is that he has removed the comments section from his blog post.
What an utterly weird story. Why not just build toilets that smell clean because they actually are? I can see why this appeals to Gates.
Biden’s Pardons were in AUTOPEN!
One of the most interesting aspects of all this is that voters finally get to discover how things work.
The executive wants to cut expenses. Does the legislative branch have to agree? Can the judiciary override both of them? One of them?
How easily funds are confiscated from the population through taxes, how easily they’re spent (squandered) and how hard it seems to roll any of that back.
Gates is the richest man in the world. He can afford to make up the loss of taxpayer largesse.
Sort of – he has given most of it away to his foundation, so technically his foundation will have to make up the loss. I guess it’s more or less the same thing.
When in doubt, Follow the Money – Pfizer-BioNTech, Moderna, Microsoft, Big Tech, etc, etc…
…Your panic, pandemic, pandemonium is our profit.
Jolly good: another brick in the Globalists’ wall being removed.
The Telegraph article was awful. When reading it, one had the impression that the USA was solely responsible for the imminent demise of GAVI. No mention of the other funders of GAVI and the high risk of conflicts of interest. Interestingly, commenting on the article was not allowed.
Remember who owns (or at least funds large parts of…) the Telegraph…
If Gates really cared about preventing disease, he would be ensuring all Africans have access to clean drinking water, and the ability to grow and transport food, these two factors are the biggest cause of death and poverty, but there is not a lot of money to be made from water or the ability to grow crops, and feed yourself
Having read such books as Virus Mania, Dissolving Illusions and The Real Anthony Fauci, I question whether any vaccine does any good whatsoever.
The simple theory is that serious illnesses are prevented by pumping a small amount of the illness (alongside with various dubious adjuvants) into your body and ‘preparing’ your body for a major attack.
But, firstly, what is the biological difference between doing that and your unvaccinated body actually experiencing an ‘attack’ of the specific illness? Secondly, what is the cumulated effect of all those poisons being pumped into my body, bypassing the body’s natural defences, when I am never, or only rarely, actually threatened by the illnesses themselves?
This guy, https://expose-news.com/2023/12/04/the-doctor-that-sacrificed-his-career-his-reputation-and-ultimately-his-life/, was certainly convinced vaccines did nothing but harm.
With the CDC recommending all children be vaccinated 81 (?) times before adulthood (https://www.cdc.gov/vaccines/hcp/imz-schedules/downloads/child/0-18yrs-child-combined-schedule.pdf), it is clear that vaccines are the major source of income for pharmaceutical companies. One wonders why anyone bothers manufacturing something cheap like aspirin.
If my memory serves me correctly, a long time ago it was claimed humans suffer from 25,000 diseases for which only 5,000 had cures. I suggest the pharmaceutical companies should be compelled to close this gap rather than propagate the use of highly dubious products, such as vaccines.
Adjuvants aka immunology’s dirty little secret.
Nobody has a clue as to precisely how and why they work save it was discovered by accident (originally they were in vaccines as preservatives), that they prolonged the desired immune response. So, quite apart from them being toxic in their own right they exhaust the immune system.
Not required for mmRNA jabs – their toxicity is long lasting without “help” from any adjuvant.
Poor African villagers will be breathing a sigh of relief at this news, after all those reports of Gates’ GAVI vaccine teams, accompanied by private armed mercenary troops, descending on African villages, and when many frightened villagers ran into the jungle with their children, they were hunted down, rounded up, and forced to have vaccines at gunpoint.