Social media has been abuzz with the publication of more than a thousand pages of FOIA’d records of the COVID-19 “Crisis Team” of the German public health authority, the Robert Koch Institute. Although heavily redacted, the records appear to show that German authorities knowingly went into a hard lockdown and adopted other draconian containment measures without scientific justification.
But there are numerous other records which German authorities hold and which could help to clarify crucial questions related to the declared COVID-19 pandemic and the response to it. Hence, there are numerous other reasons for testing the waters of Germany’s relatively recent Freedom of Information law. (Germany’s Freedom of Information Act dates from 2005 and was revised in 2013.)
For instance, with increasing attention being paid to a recent explosion of cancer diagnoses and everyone but the mainstream media wondering if it might not be connected to oncogenic properties of the mRNA vaccines with which virtually the entire Western world was vaccinated in the name of combatting COVID-19, would it not be interesting to know more about earlier clinical trials of the German company BioNTech?
As at least my readers will know, BioNTech is the actual owner and legal manufacturer of what is more commonly and misleadingly known as the “Pfizer” COVID-19 vaccine. It is also a company which was founded to develop mRNA-based cancer treatments and which was almost entirely focused on this goal prior to Covid. The company’s founders, Ugur Sahin and Özlem Türeci, are oncologists.
Since its founding in 2008, however, BioNTech had not gotten very far. In The Vaccine, the auto-hagiographical account of the development of their COVID-19 vaccine which Sahin and Türeci wrote with Financial Times journalist Joe Miller, they say that they had tested their mRNA-based cancer drugs on merely 400 people in Phase 1 and Phase 2 clinical trials since 2012 (p. 41). Why had they never made it to a large-scale Phase 3 clinical trial?
A search of the EU Clinical Trials Register turns up just three Phase 2 BioNTech clinical trials which were registered prior to 2020. (Phase 1 trials involving only adults are not included in the database.) No results are available for any of them. The earliest of these trials only began in 2019, just five months before the official start of the Covid outbreak in Wuhan. This trial, however, is listed as having been “prematurely ended”. See the screenshot below. (“HU” stands for Hungary and “DE” for Germany.)
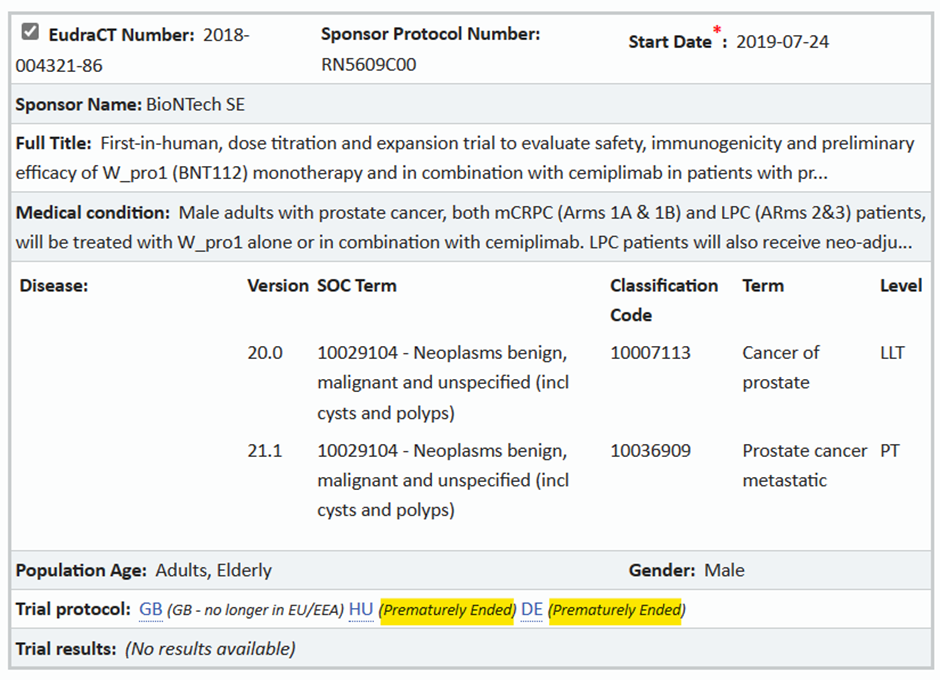
Why did BioNTech’s cancer drug trials not get any further? Could it have been that the company’s mRNA-based therapies were in fact accelerating cancers rather than reversing them?
As the responsible oversight authority, the German vaccines and medicines regulator, the PEI, certainly knows and possesses information which could help clarify these questions. Moreover, as I have shown here, the PEI has in fact a longstanding collaborative relationship with the company.
Think of the massive amount of documentation which American courts have forced the FDA to release on the clinical trial of BioNTech’s COVID-19 vaccine (the so-called “Pfizer documents”, even though the sponsor of the trial in question was in fact BioNTech). Why should the PEI not be required to be just as forthcoming about BioNTech’s prior cancer drug trials?
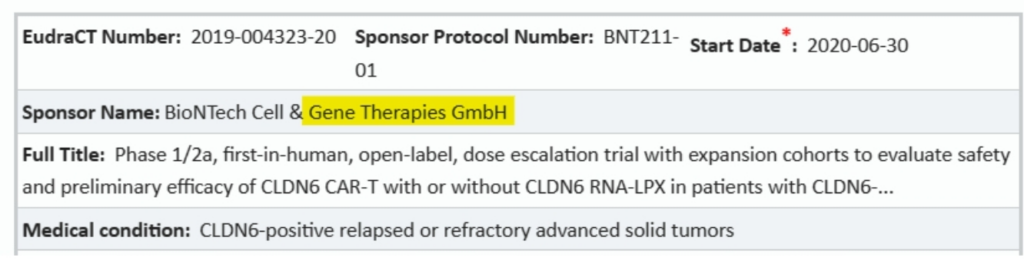
Stop Press: By the way, the third of the Phase 2 clinical trials registered by BioNTech prior to 2020 is also of some interest. This one was registered in 2019. Look at how the sponsor name is given.
Robert Kogon is the pen name of a widely-published journalist covering European affairs. Subscribe to his Substack.














To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
There’s that word Neoplasm again. Andrew Bridgen released NHS data about a week* ago showing that since 2021 there has been a rapid rise in the diagnosis of ‘benign neoplasm brain infratentorial’, especially among the young.
Just a coincidence I guess….
*Also in Melissa Kite article yesterday
I can’t help but wonder whether the cocktail of chemicals people shoved up their noses every day for the stupid lateral flow tests (single use plastic perfectly ok for these by the way!) has anything to do with these brain issues.
with increasing attention being paid to a recent explosion of cancer diagnoses and everyone but the mainstream media wondering if it might not be connected to oncogenic properties of the mRNA vaccines
I am well aware of the sharp rise in cancer among the young over the last two decades – but what evidence is there of an “explosion” in cancer since the vaccines?
From Melissa Kite’s article yesterday https://twitter.com/ABridgen/status/1771104346270208484/photo/1
My own anecdotal experience : 5 friends with various new cancer diagnoses since 2022 (all of them self-proclaimed vax enthusiasts in the early days.
I can’t read the detail of the Bridgen tweet but I think it is about illness in general not cancer. I am sorry about your friends but that is anecdotal evidence. This data (click on data download on the LHS) shows that while there was a sharp increase in excess cancer deaths during Covid there has been almost no excess deaths due to cancer since the vaccination programme – of course deaths are not the same as diagnosis and it would be interesting to see actual data for cancer diagnosis since 2021.
From this oncologist, who was a former vaccine-enthusiast
https://www.canceractive.com/article/top-professor%20sees%20vaccines%20causing%20cancer
Where are the data?
It’s a good question – cancer diagnosis since 2019 has not been published – why not? I refuse to believe the ONS cannot obtain it, so why are they only showing the old data and not updating it?
I think it just takes time. Also can be hard to find the data in the right form for our particular question. I just found there are statistics for 2021: https://digital.nhs.uk/data-and-information/publications/statistical/cancer-registration-statistics – (they just show that incidence returned to preepidemic levels)
Then you answered your own question (‘Where are the data?’).
However, I would believe an actively practising oncologist who is saying he has seen a huge upsurge in cancer since the vaccine rollout. Would you not?
Yes. I found some data and it shows no cancer explosion.
I believe the oncologist personal experience but that is just what happened to him (or her). There are at least two things to take into account.
1 During 2020 cancer diagnosis was much lower than usual (see my data above). The obvious explanation is that people with symptoms were reluctant to go to the doctor so they were not diagnosed. This inevitably led to an apparent increase when the epidemic died and diagnoses caught up.
2 There must be many thousands of oncologists and by sheer chance a few of them will have seen a surge in cases in 2021/22. Once their suspicion is aroused they will take particular note of colleagues who support their story, especially in the light of 1.
What I am looking for is solid data not anecdotes, even from oncologists.
Dr John Campbell (Medical lecturer) here is reasonably pointing out that if if the data does not show an increase in cancers since 2021, then why aren’t they releasing it?
https://www.youtube.com/watch?v=mxBz-jHDy_w
The data I linked to in this earlier comment shows there was no increase in cancer in 2021 in the UK – so if the vaccinations have an effect it takes time or requires multiple jabs!
The report came out in Oct 23 so it seems it takes quite a long time to get solid cancer diagnosis statistics (no idea why). We can maybe look forward to the 2022 data about Oct this year.
It’s as plain on the nose on your face, MTF. Do you remember such a hike in cancer rates among the young before the jab? And let’s not call them ‘vaccines’. They never were ‘vaccines’, they were an experimental type of gene therapy.
Eh? There have been many stories about the surge in cancer rates among the young over the period 1990 to 2019 (including two on DS) based on this study – none that I know of about cancer cases since 2021.
That screenshot:
We were given very specific instructions after the last cataclysm and it was sealed with the covenant of a rainbow. That the world would not be detroyed again by water. Before the last cataclysm they started conducting all sorts of nasty genetic experiments. DNA is like a cosmic radio transmitter. If you mess with things in higher dimensions then expect higher dimensional consequences. If you look at UFO crash sites they tended to be focussed around nuclear explosions. The point is that your detonations destroyed something in their world. These entitities were often reported to be found to be crying or upset.
If you have grown up with Captain Kirk thinking that science is to boldly go where no man has gone before then it is great that you have Promeathean energy. There aren’t limits on where you are allowed to go but if you launch a challenge to the spiritual world from the material then you create a very difficuly situation. You need to have applied yourself to the greater understanding first otherwise your technology will merely represent an attack and an affront and it won’t last long. If you lack the depth to do that tthen you certainly shouldn’t be anywhere near public policy. Obviously the events of the last five years have thrown this into stark relief. Apocalypto baby – the lifting of the veil. If you have a pulse then you should rejoice in just how much is being laid bare.