The MHRA’s reports on the COVID-19 vaccines, which are updated in line with its summary of the Yellow Card reporting publication. The MHRA Summary of Yellow Card Scheme Reporting for COVID-19 vaccines was published on March 8th 2023.
Since January, the reports have focused on the vaccines from the beginning of the Autumn 2022 booster campaign.
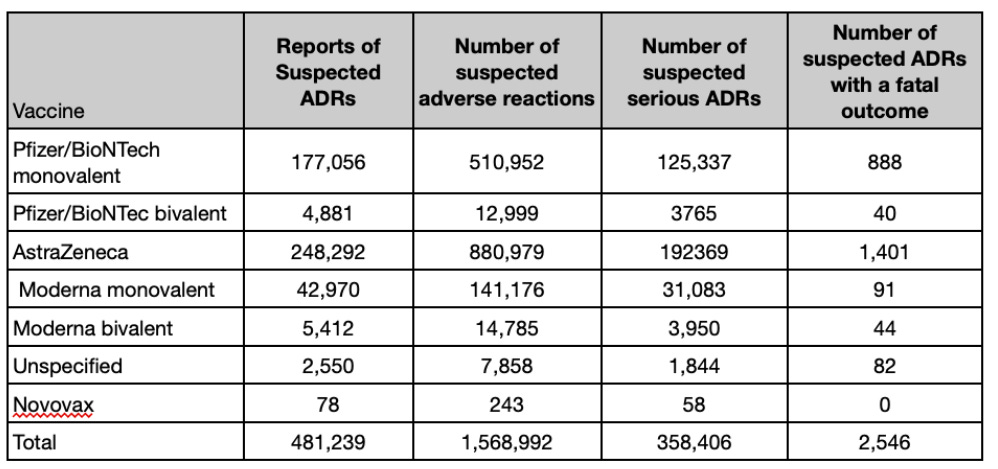
As of February 22nd 2023 in the U.K., 4,096 Yellow Card events were reported for the bivalent COVID-19 vaccine Pfizer/BioNTech, 5,108 for the bivalent COVID-19 vaccine Moderna, 57 for the COVID-19 vaccine Novavax and 2,319 were reported where the brand of the vaccine was not specified.
The MHRA had received 30 U.K. reports of suspected adverse drug reactions (ADRs) with a fatal outcome to the bivalent COVID-19 Pfizer/BioNTech vaccine and 42 fatal reports of suspected ADRs with the bivalent COVID-19 vaccine Moderna. The MHRA received no U.K. reports of a fatal outcome for the COVID-19 vaccine Novavax.
Interactive reports for each vaccine can be found here:
- COVID-19 Vaccine Pfizer/BioNTech monovalent
- COVID-19 Vaccine Pfizer/BioNTech bivalent
- COVID-19 Vaccine AstraZeneca
- COVID-19 Vaccine Moderna monovalent
- COVID-19 Vaccine Moderna bivalent
- COVID-19 Vaccine – brand unspecified or not in routine use in the U.K.
- COVID-19 Vaccine Novavax
It is not clear, though, why the reports focus on the ADRs since the booster campaign.
The full analysis reports substantially more data, with nearly half a million reports of suspected ADRs for the Covid vaccines and 2,546 suspected ADRs with a fatal outcome.
The reports are likely to be an underestimate of the actual number of suspected adverse reactions.
The MHRA considers its previous estimates of underreporting (that only 10% of serious reactions are reported) should not be used as indicators of the reporting rate for COVID-19 vaccines, as it considers there is high public awareness of the Yellow Card scheme. There is no evidence of this heightened awareness, and it is plausible, given the previous estimate, that the number of suspected adverse reactions could be 10-fold higher than the number reported. Given the seriousness of the issues, it is unclear why the MHRA does not perform validation studies to ascertain a more robust estimate.
The MHRA says it takes all reports with a fatal outcome in patients who have received a COVID-19 vaccine very seriously, and every report with a fatal outcome is reviewed carefully.
However, the MHRA does not attempt to assess or compare the safety of different vaccines. This occurs because it uses inadequate reporting in the system to prevent any analysis.
It is not possible to compare the safety of different vaccines by comparing the numbers presented in the vaccine reports. Reporting rates can be influenced by many factors, including the seriousness of the adverse reactions, their ease of recognition and the extent of use of a particular vaccine. Reporting can also be stimulated by promotion and publicity about a product.
The information for healthcare professionals and the recipient provides a list of the recognised adverse effects (see here).
Systems across other countries are better at identifying adverse reactions to vaccines. Health authorities in Denmark, Norway and Iceland suspended the use of AstraZeneca’s COVID-19 vaccine in March 2021 following reports of the formation of blood clots, while the European medicine regulator reported the vaccine’s benefits outweighed its risks and could continue to be administered.
On April 7th, the U.K. JCVI advised the AstraZeneca vaccine should be restricted to people aged 30 and over because of the risk of blood clots.
Yet, at the same time, the MHRA was “not recommending age restrictions in COVID-19 AstraZeneca vaccine use”. The MHRA’s scientific review of U.K. reports of blood clots with lowered platelets concluded the evidence of a link with AstraZeneca’s vaccine was stronger, but more work was still needed.
On May 7th, Britain restricted AstraZeneca to people aged over 40. However, within a week, Norway permanently removed AstraZeneca from its vaccine programme, and several countries followed suit.
The Norwegian Medicines Agency publishes weekly overviews of suspected adverse reactions associated with COVID-19 vaccination. Furthermore, in Norway, ADR reporting has been defined in legislation: pharmaceutical companies, doctors and dentists are obliged to report on severe or unexpected ADRs, and healthcare staff and patients are recommended to report ADRs spontaneously.
Influence of industry
In 2005, the House of Commons Health Committee reported on the influence of the pharmaceutical industry. At the time:
The MHRA was unusual in being one of few European agencies where the operation of the medicines regulatory system was funded entirely by fees derived from services to industry (drug regulatory agencies in other countries are more often only partly funded by licence fees). The MHRA’s activities are 60% funded through licensing fees paid by those seeking marketing approvals and 40% through an annual service fee, also paid by the industry.
The committee reported that the MHRA had “failed to adequately scrutinise licensing data and its post-marketing surveillance is inadequate”.
The MHRA continues to be primarily funded by income from fees for sales of products and regulatory services: the breakdown sees 50% fees for services, 25% industry periodic fees and 25% department funding.
Improving the reporting of suspected adverse drug reactions
In Wales, Yellow Cards submitted by GPs more than doubled with the introduction of a National Reporting Indicator in 2014. Reporting rates continued to increase yearly through 2018-19, with the NRI still in place.
Also, in Wales, a study including 1,606 public members reported nearly half had previously experienced an ADR. Before the educational video, 18% knew how to report an ADR via the Yellow Card System immediately after watching it, 71% reported knowing how to report, and 82% reported being confident.
A guide for children and young people has also been shown to inform them how to report a suspected ADR to the MHRA and increase their knowledge and confidence in reporting.
However, one of the major barriers to public participation in ADR reporting remains uncertainty about the legitimacy of involvement in the Yellow Card Scheme and doubts about the value of the information provided.
A survey of U.K. pharmacists suggested they lack interest in and do not promote direct patient reporting. Only 19% of the respondents displayed a poster promoting the Yellow Card Scheme in their pharmacy.
In 2020, the MHRA published what it will do differently, identifying four main themes:
- Awareness: Levels of public awareness of the agency and its role and responsibilities are still relatively low, although higher than when we previously surveyed stakeholders and patients some years ago.
- Transparency: There is a perceived lack of transparency about how the agency makes its decisions and the information that it currently provides.
- Responsiveness: There is a lack of responsiveness from the agency when concerns are raised, especially by patients, who say they often do not feel listened to and that the agency’s response is not always proportionate to the seriousness of patients’ issues.
- Partnership: (including patient and public involvement) The development of partnerships with stakeholders, including the involvement of patients in the agency’s decision-making process, as well as further development/use of digital communication channels, will be key to addressing the issues around awareness, transparency and responsiveness.
The MHRA also published a proposed ‘Patient and Public Involvement Strategy 2020-25‘, aiming to adopt a more systematic approach to listening to and meaningfully involving patients and the public. However, the strategy lacks specifics about improving patient safety and performance metrics. For example, the MHRA’s strategy states one of the ways to achieve its objective is to improve the user experience of the Yellow Card scheme — nothing on under-reporting, analysis of signals or assignment of causation.
Conclusions
There are widespread problems with the reporting of adverse drug, biologics and device reactions, which substantially compromises patient safety.
Adverse drug reactions are a major cause of hospital admission, a major concern for inpatients and a considerable burden on the quality of life for those afflicted, leading to death in the most severe cases.
The accumulating evidence and the IMMDS review (the Cumberlege report) showed the MHRA’s approach to patient safety requires a radical overhaul.
Patient reporting can bring novel, meaningful information about ADRs, complementing information from healthcare professionals. However, it is rare, and the level of underreporting is substantial – as many as 98 out of every 100 ADRs go unreported. The problems in the system have been overseen by the MHRA, which has failed in its remit to keep patients safe. Conflicts beset the system, and it is often too late to act to detect serious harm. The cost to the health system of adverse drug and device reactions is substantial, and failure to act will only lead to more harm.
The pervasive problem with identifying adverse severe drug, biologic and device reactions requires a parliamentary select committee to make recommendations that encompass legislative changes as to who is obliged to report adverse reactions, funding changes to the MHRA, separation of regulatory approval duties from post-marketing pharmacovigilance, and more inclusion of patients to improve the current system.
Prof. Carl Heneghan is the Oxford Professor of Evidence Based Medicine and Dr. Tom Jefferson is an epidemiologist based in Rome who works with Professor Heneghan on the Cochrane Collaboration. This article was first published on their Substack, Trust The Evidence, which you can subscribe to here.













To join in with the discussion please make a donation to The Daily Sceptic.
Profanity and abuse will be removed and may lead to a permanent ban.
We live in the upside down and the state now exists to censor any debate about topics such as vaccine safety, transgenderism etc. Very clearly the intelligence agencies are now spying on their citizens to such an extent that they are completely out of control imposing a narrative that they see as liberal. They are also happy to weaponised narratives such as COVID and the Ukraine war against their own citizens. Until such times as these agencies are scrapped things will not return to normal.
I compiled the attached table of Covid-19 ‘Vaccine’ Adverse Effects in November 2022, with data taken from UK, EU and USA databases. The total number of registered fatalities was then more than 83,000, never mind the countless hundred thousand other unpleasantries.
It was well known at that time that medicines with ‘only’ double-digit fatal outcomes had had their authorization withdrawn, so there was/is no excuse to continue distribution of drugs with fatalities in their thousands.
There can be no denying that disregarding these data was, and is, wilful.
Explain to a normie that the jabs have killed 2500 and they’ll laugh in your face, then explain that this is possibly only 10% you’re a nut job they’ve saved thousands in the UK alone. I’ve had 25,000 deaths as a baseline from the yellow card reporting, Prof Fenton has estimated 50,000. With excess deaths running at there current rate we’re talking over a 100,000 and there’s plenty more to come, add to that the infertility this is going to have a catastrophic societal effect.
Every day you here stories of heart attacks cancer, neurological issues and death, I’ve given up telling people it’s the vaccine they’re to entrenched. I’ve almost given up reading about this horror story what more is there we need to know about the side effects the list is already to long. Depopulation anyone where’s Hux when you need him.
Just a quick update. These figures are from July 2023. The latest figures published a few days ago up to 27th December 2023 are attached. I group by age here, but more details are at https://t.me/mikes_stuff/1402
In many, more normal, industries, recording of relatively minor errors are considered to be useful, rather than only dealing with severe outcomes. This is based on the idea that it is potentially useful as a method of avoiding worse outcomes. As suggested by others, it does look as if the pharmaceutical industry is in an upside down world.